PATENTS
Patent Applications Filed:
4. Papish, E. T.; Paul, J. J.; Merino, E. J. “pH and Light Activated Anti-Cancer Drugs” provisional patent filed by the University of Alabama at USPTO in 10/14/2015, full patent application # 20160101177 A1 filed 4/14/2016.
Patents Granted:
3. Papish, E. T.; Vannucci, A. K. “Selective Hydrodeoxygenation of Aromatic Compounds” United States Utility Patent No. 11,773,040 B2, granted 10/3/23, filed on 12/30/19.
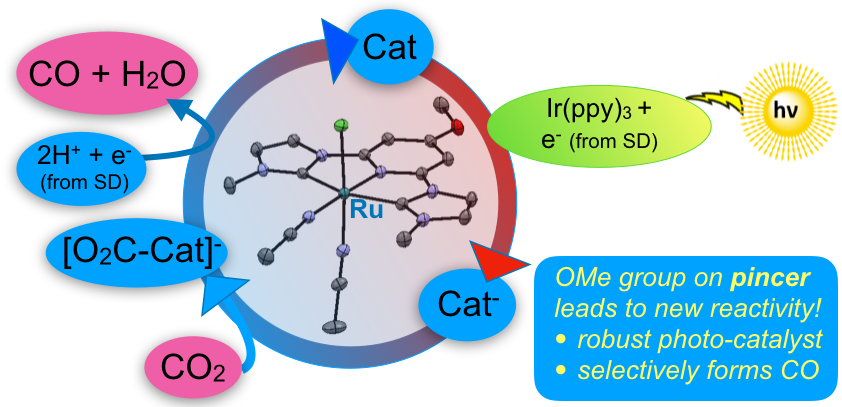
2. Papish, E. T.; Delcamp, J. H. “Light Driven Metal-Pincer Photocatalysts for Carbon Dioxide Reduction to Carbon Monoxide” United States Patent 11,103,861, granted August 31, 2021, filed September 15, 2017.
1. Papish, E. T.; Nieto, I. “Dihydroxybipyridine Complexes of Ruthenium and Iridium for Water Oxidation and Hydrogenation” United States Patent 9,527,066 B2, Dec. 27, 2016. (Provisional application filed 8/30/11, patent application filed in 2/28/14.) Link to USPTO site for this patent.
PAPERS (* denotes undergraduate coauthors)
Google scholar profile: H index = 23. Papers with 10 citations or more: 40.
The following publications are from work at the University of Alabama:
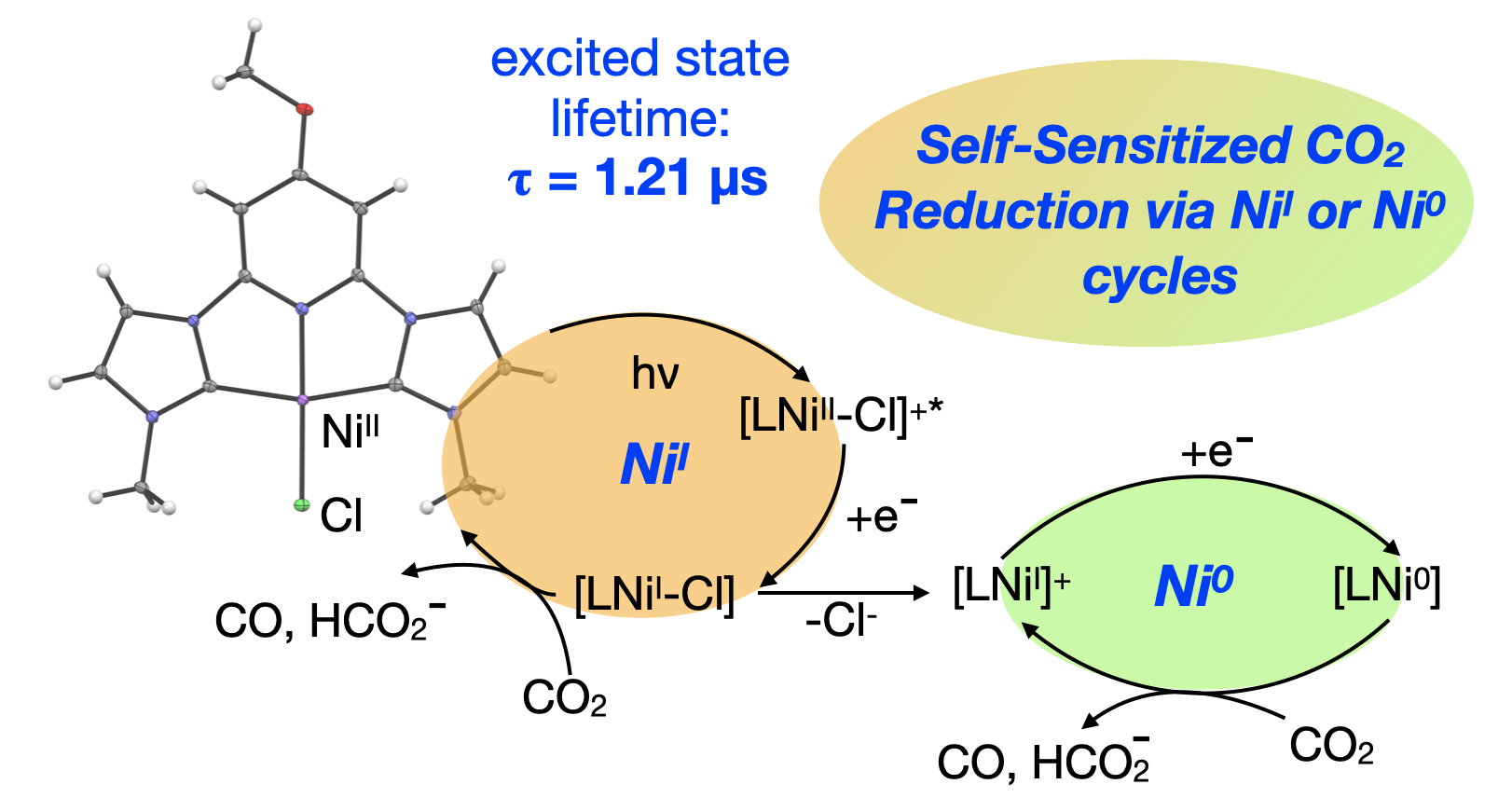
63. Manafe, S. Y.; Le, N.; Lambert, E. C.; Curiac, C.; Nugegoda, D.; Das, S.; Hunt, L. A.; Qu, F.; Whitt, L. M.; Fedin, I.; Hammer, N. I.; Webster, C. E.; Delcamp, J. H.; Papish, E. T. “Sensitized and Self-sensitized Photocatalytic CO2 Reduction to HCO2– and CO under Visible Light with Ni(II) CNC-Pincer Catalysts” ACS Catal., 2024, accepted 3/27/24, in press. https://doi.org/10.1021/acscatal.3c03787
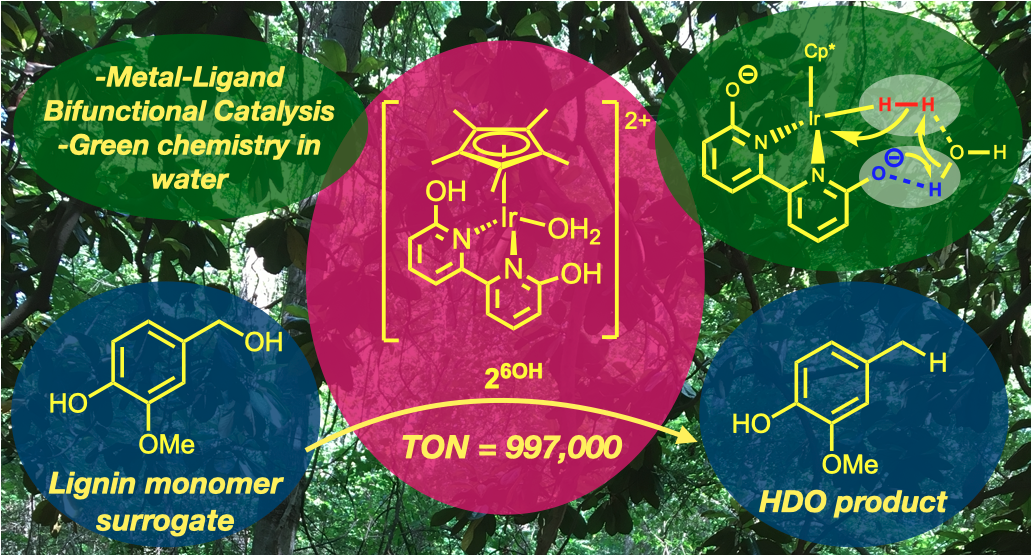
62. Yao, W.; Buell, C. A.; Kuppravalli, A.; Vannucci, A. K.; Papish, E. T. “Iridium Dihydroxybipyridine Complexes are Effective Catalysts for Hydrodeoxygenation of Vanillyl Alcohol in Water” Organometallics, 2023, 42, 2806–2812. https://doi.org/10.1021/acs.organomet.3c00273
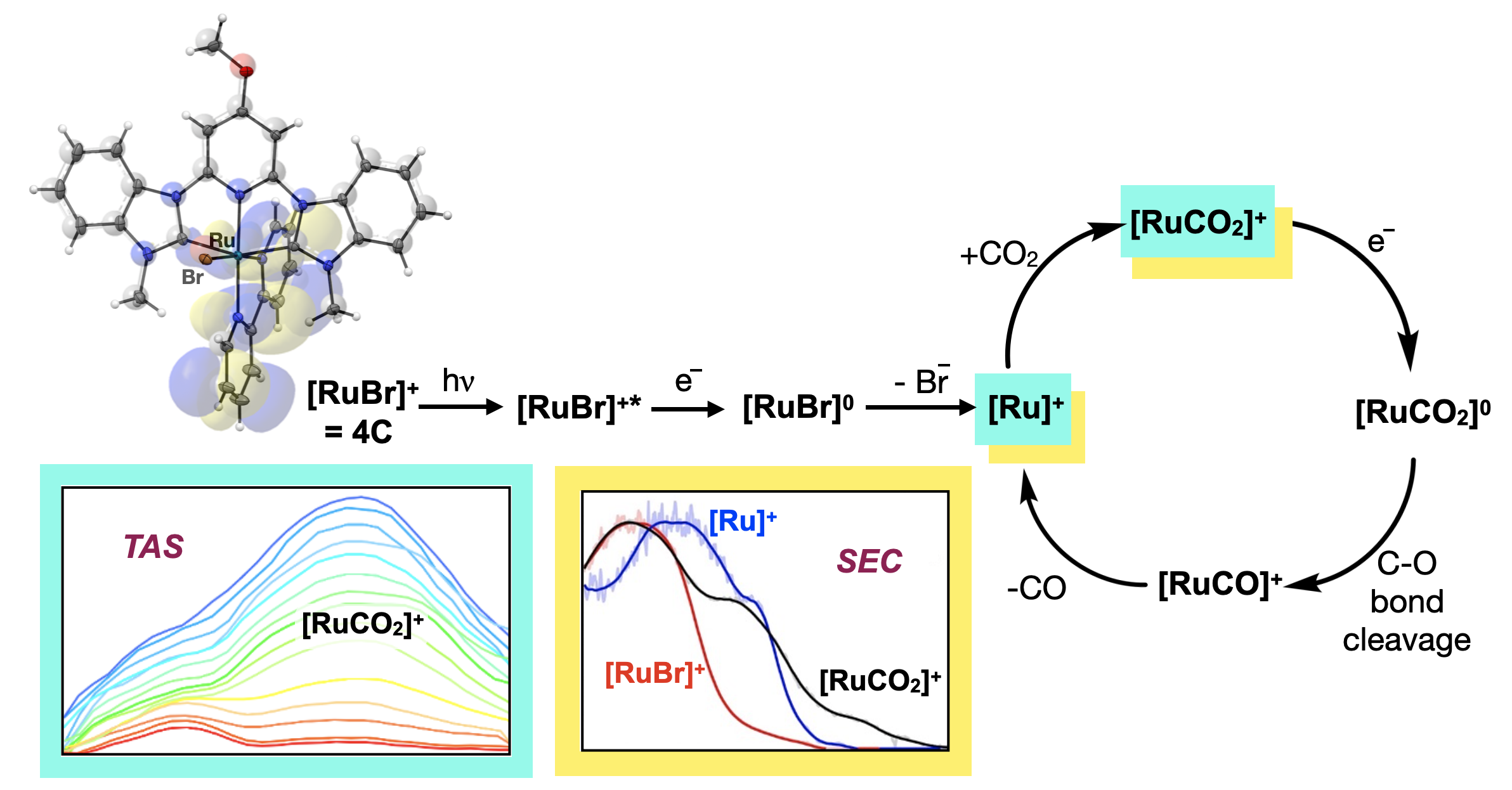
61. Hunt, L. A.;† Das, S.;† Lamb, R. W.;† Nugegoda, D.; Curiac, C.; Figgins, M. T.;* Lambert, E. C.;* Qu, F.; Hammer, N. I.; Delcamp, J. H.; Webster, C. E.; Papish, E. T. “Ruthenium (II) Complexes of CNC Pincers and Bipyridine in the Photocatalytic CO2 Reduction Reaction to CO using Visible Light: Catalysis, Kinetics, and Computational Insights” ACS Catal. 2023, 13, 5986–5999. https://doi.org/10.1021/acscatal.2c05459
†Contributed equally
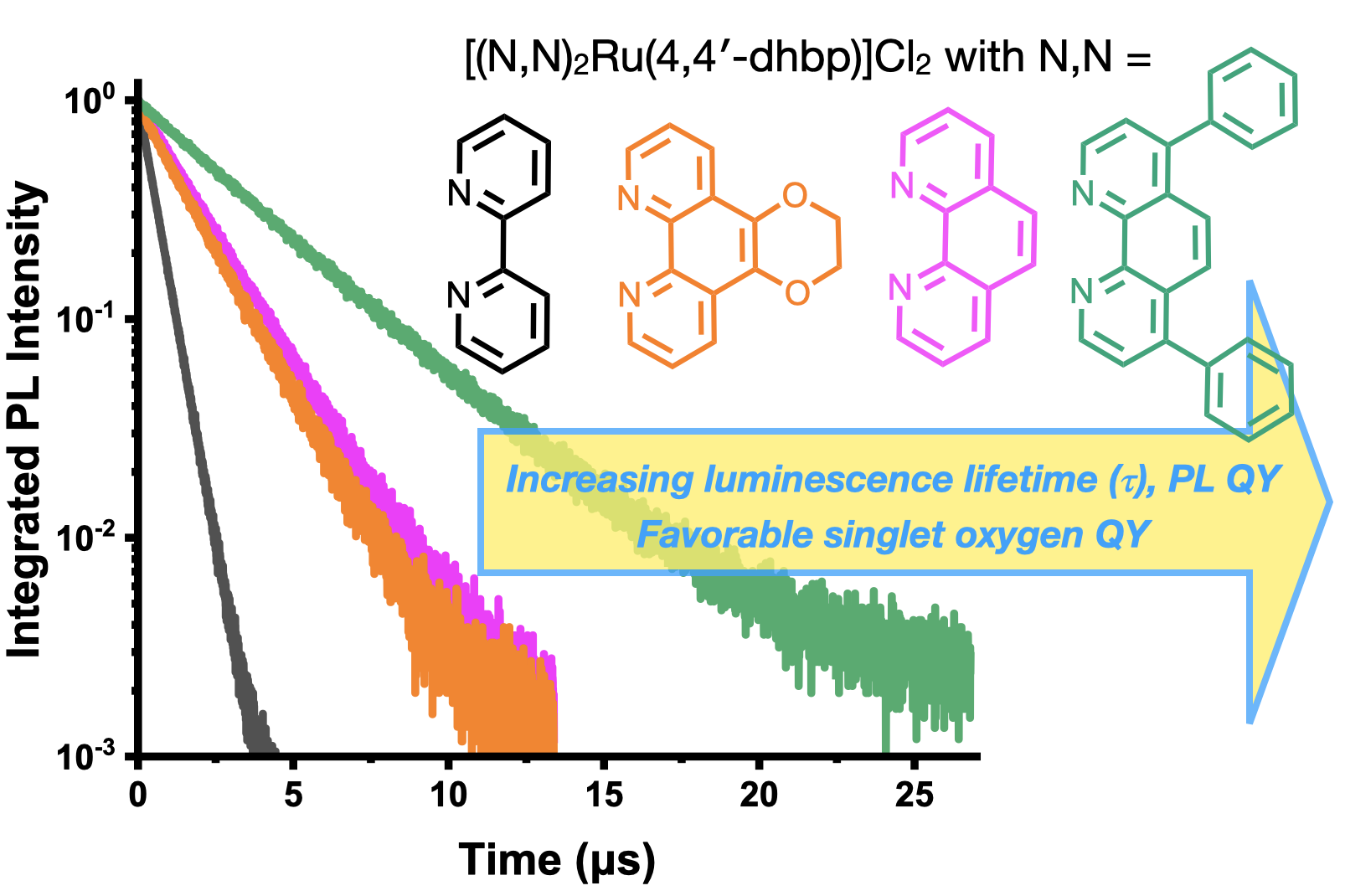
60. Oladipupo, O. E.; Prescott, M. C.; Blevins, E. R.; Gray, J. L.; Cameron, C. G.; Qu, F.; Ward, N. A.; Pierce, Abigail L.; Collinson, E. R.; Hall, J. F.; Park, S.; Kim, Y.; McFarland, S. A.; Fedin, I.; Papish, E T. “Ruthenium Complexes with Protic Ligands: Influence of the Position of OH Groups and π Expansion on Luminescence and Photocytotoxicity” Int. J. Mol. Sci. 2023, 24(6), 5980. For invited issue on “Anticancer Drug Design and Development”. https://www.mdpi.com/1422-0067/24/6/5980
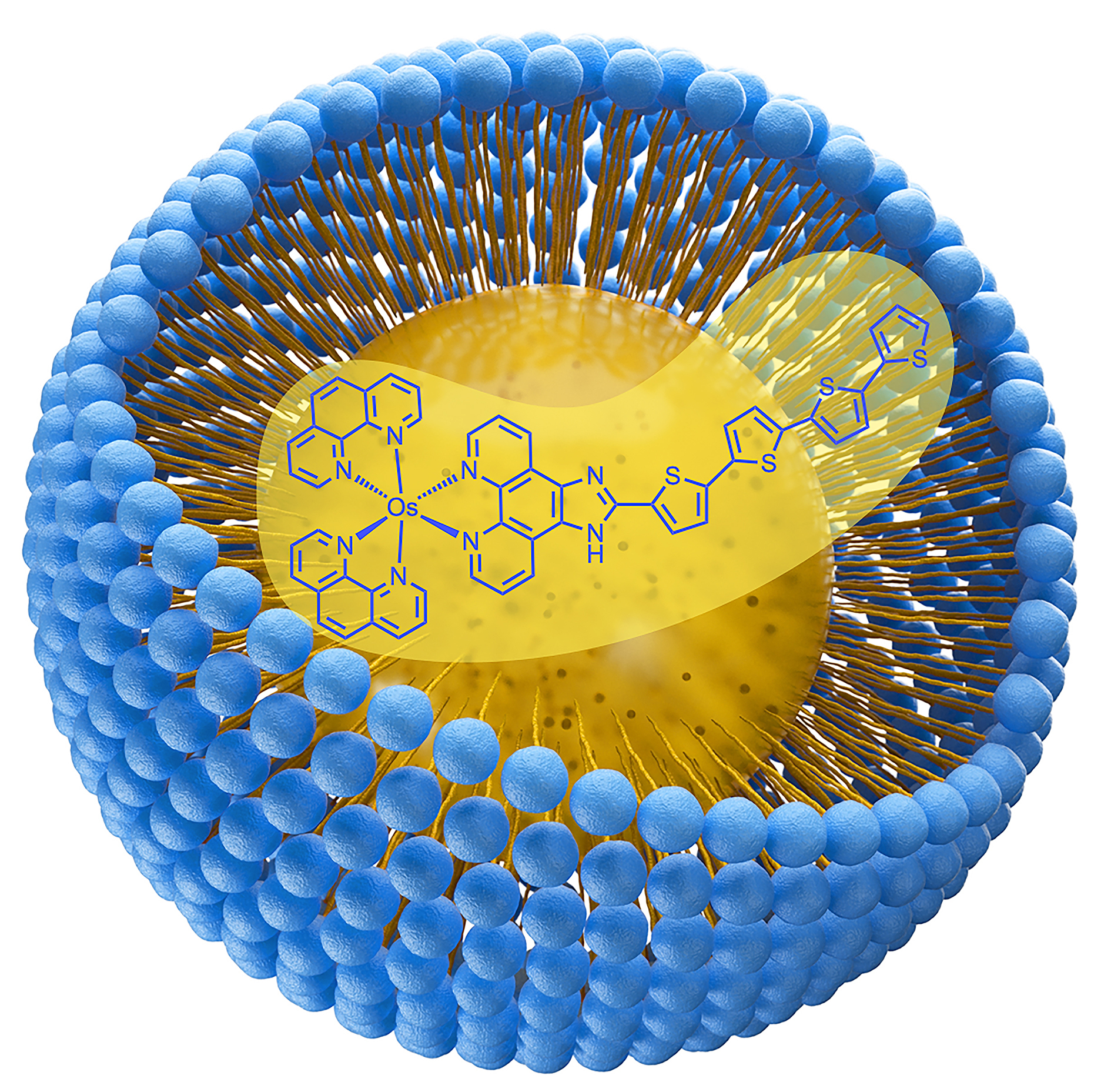
59. Papish, E. T. “Nanoformulation of Lipophilic Osmium Photosensitizers in Liposomes and Micelles as a General Strategy for Improving Reproducibility and Reducing Quenching” Photochemistry and Photobiology, 2023, invited highlight article submitted 12/7/22, accepted 12/27/22. https://doi.org/10.1111/php.13776
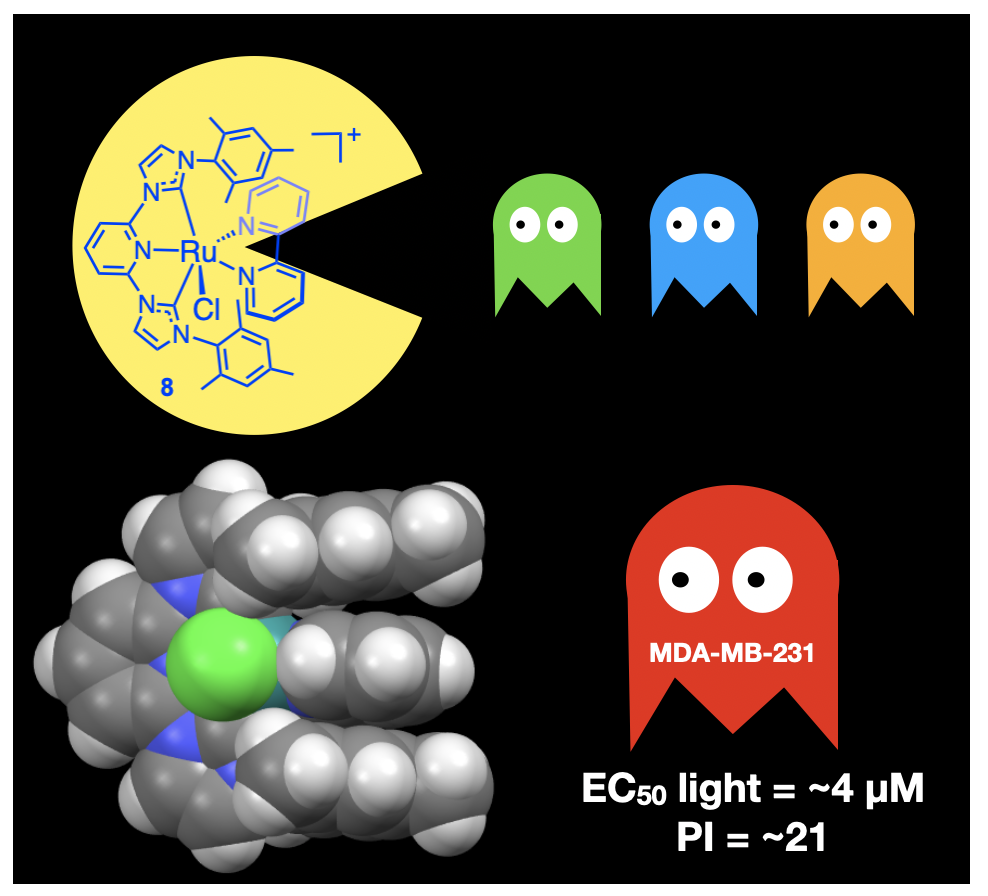
58. Sun, Y.*; Das, S.; Brown, S. R.; Blevins, E. R.*; Qu, F.; Ward, N. A.*; Gregory, S. A.*; Boudreaux, C. M.; Kim, Y.; Papish, E. T. “Ruthenium Pincer Complexes for Light Activated Toxicity: Lipophilic Groups Enhance Toxicity” J. Inorg. Biochem. 2023, 240, 112110. DOI: 10.1016/j.jinorgbio.2022.112110
https://www.sciencedirect.com/science/article/pii/S0162013422003993?via%3Dihub
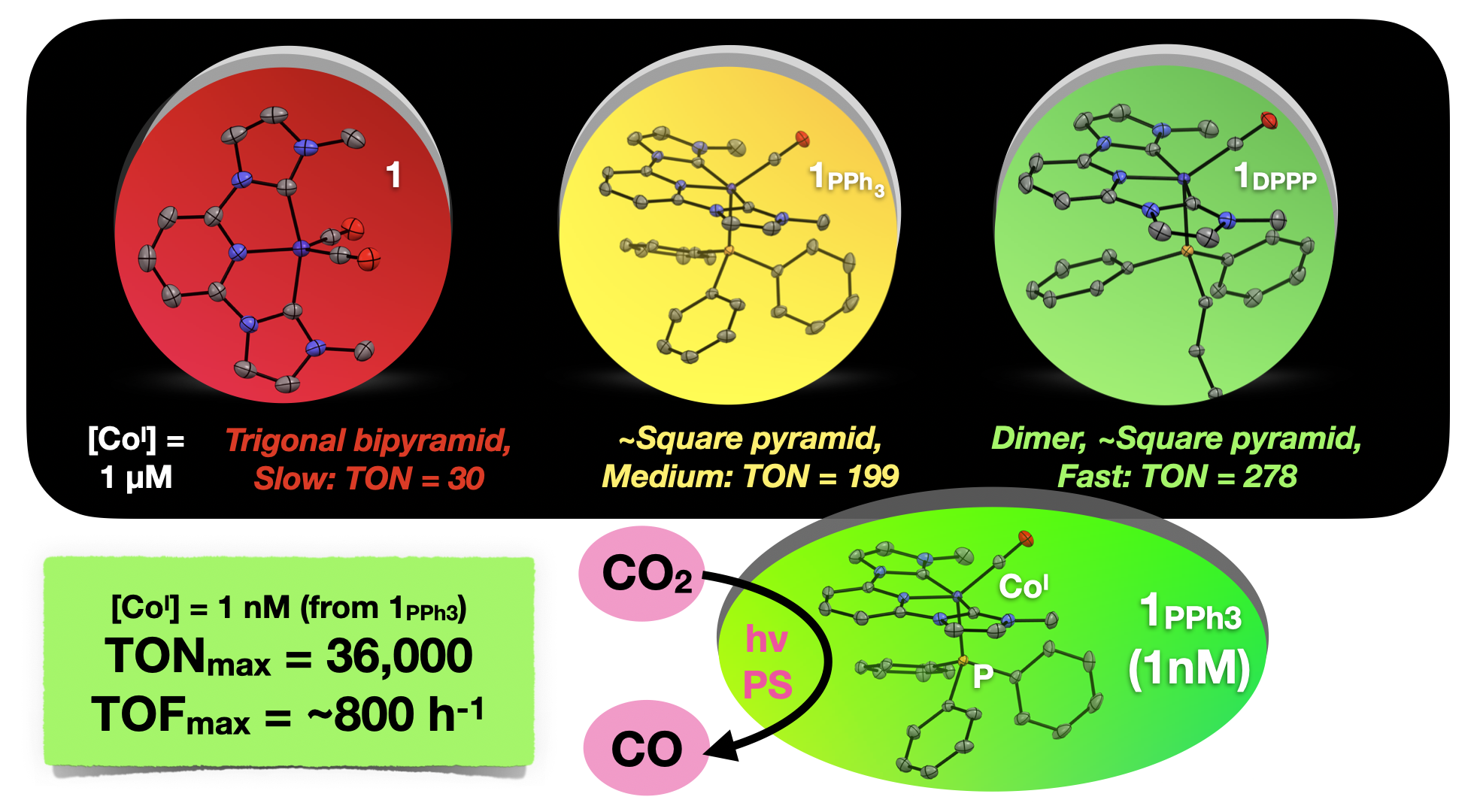
57. Boudreaux, C. M.; Nugegoda, D.; Yao, W.; Le Tri, N.; Frey, N. C.*; Li, Q.*; Qu, F.; Zeller, M.; Webster, C. E.; Delcamp, J. H.; Papish, E. T. “Low Valent Cobalt(I) CNC Pincer Complexes as Catalysts for Light Driven Carbon Dioxide Reduction” ACS Catalysis, 2022, 12, 8718−8728. https://pubs.acs.org/doi/abs/10.1021/acscatal.2c01281
56. Das, S.; Nugegoda, D.; Yao, W.; Qu, F.; Figgins, M. T.*; Lamb, R. W.; Webster, C. E.; Delcamp, J. H.; Papish, E. T. “Sensitized and Self-Sensitized Photocatalytic Carbon Dioxide Reduction Under Visible Light with Ruthenium Catalysts Shows Enhancements with More Conjugated Pincer Ligands” Eur. J. Inorg. Chem., 2022, published online 1/10/22. DOI: 10.1002/ejic.202101016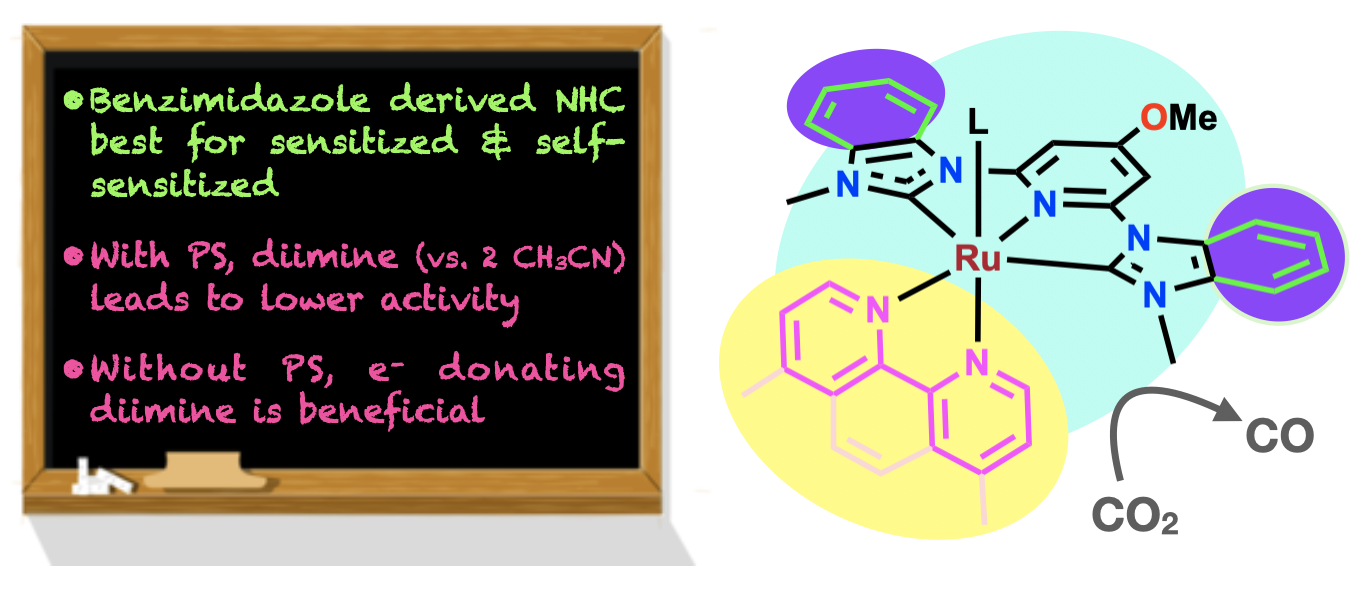
55. Papish, E. T.; Oladipupo, O. E. “Factors that Influence Singlet Oxygen Formation vs. Ligand Substitution for Light Activated Ruthenium Anticancer Compounds” Current Opinion in Chemical Biology 2022, 68, 102143. https://doi.org/10.1016/j.cbpa.2022.102143
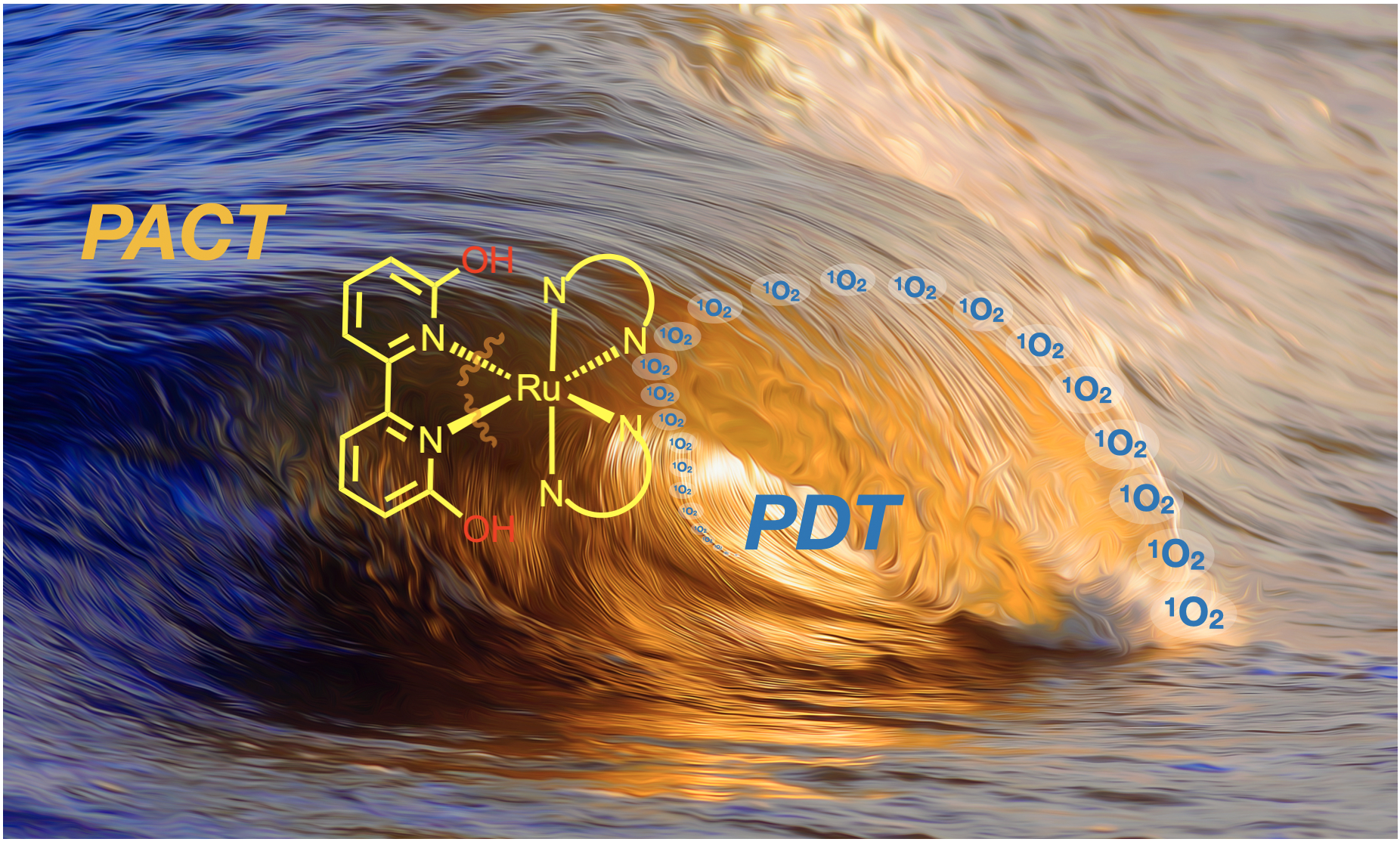
54. Oladipupo, O.; Brown, S. R.; Lamb, R. W.; Gray, J. L.; Cameron, C. G.; DeRegnaucourt, A. R.*; Ward, N. A.*; Hall, J. F.*; Xu, Y.; Petersen, C. M.; Qu, F.; Shrestha, A. B.; Thompson, M. K.; Bonizzoni, M.; Webster, C. E.; McFarland, S. A.; Kim, Y.; Papish, E. T. “Light-responsive and protic ruthenium compounds bearing bathophenanthroline and dihydroxybipyridine ligands achieve nanomolar toxicity towards breast cancer cells” Photochem. and Photobiol. 2022, 98, 102-116. For special issue in memoriam of Prof. Karen Brewer. https://onlinelibrary.wiley.com/doi/abs/10.1111/php.13508
53. Papish, E. T.; Das, S.; Silprakob, W.; Boudreaux, C. M.; Manafe, S. Y. “Proton Responsive and Hydrogen Bonding Ligands in Organometallic Chemistry” invited book chapter for Comprehensive Organometallic Chemistry IV, 2022, 1, 442-473. https://www.sciencedirect.com/science/article/pii/B9780128202067000809
52. Qu, F.; Lamb, R. W.; Cameron, C. G.; Park, S.; Oladipupo, O.; Gray, J. L.; Xu, Y.; Cole, H. D.; Bonizzoni, M.; Kim, Y.; McFarland, S. A.; Webster, C. E.; Papish, E. T. “Singlet oxygen formation vs. photodissociation for light-responsive protic ruthenium anticancer compounds: The oxygenated substituent determines which pathway dominates” Inorg. Chem. 2021, 60, 2138–2148. DOI: http://dx.doi.org/10.1021/acs.inorgchem.0c02027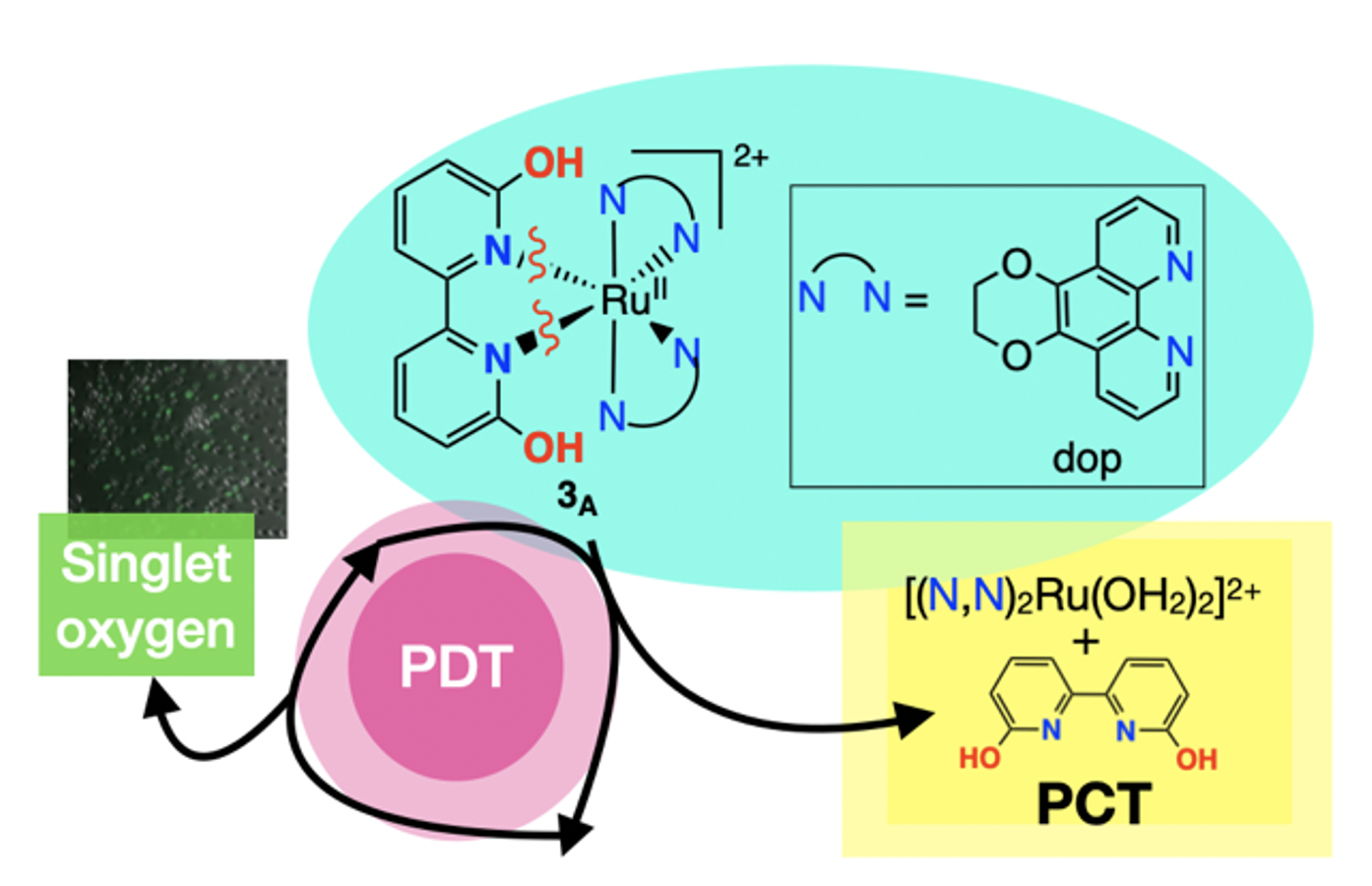
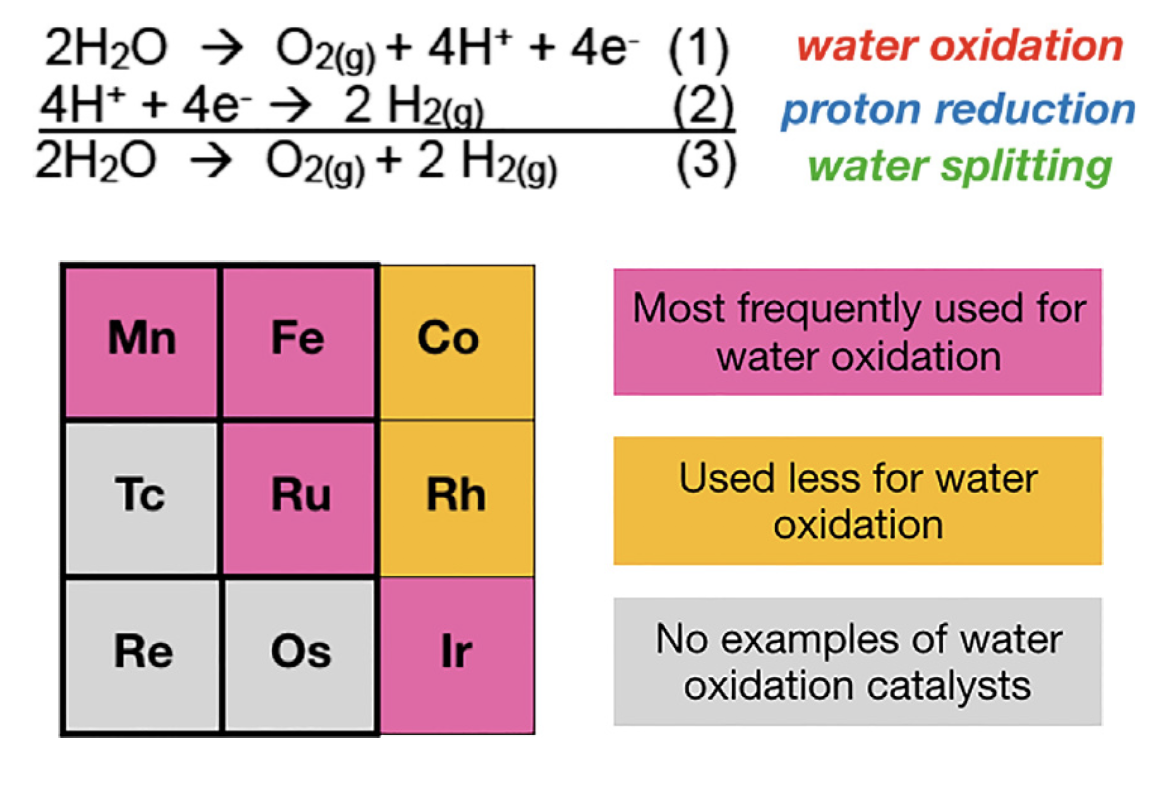
51. Papish, E. T. “Water Oxidation with Coordination Complex Catalysts Using Group 7 and 8 Metals” invited book chapter for Comprehensive Coordination Chemistry III, 2021, 715-741. https://doi.org/10.1016/B978-0-12-409547-2.14688-8
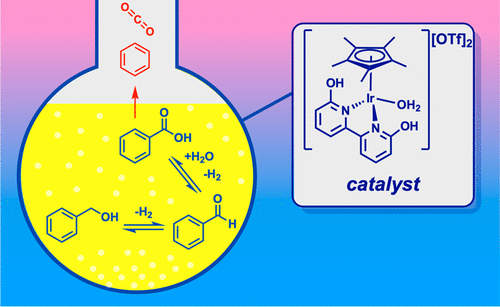
50. Yao, W.; DeRegnaucourt, A. R.*; Shrewsbury, E. D.*; Loadholt, K.* H.; Silprakob, W.; Brewster, T. P.; Papish, E. T. “Reinvestigating Catalytic Alcohol Dehydrogenation with an Iridium Dihydroxybipyridine Catalyst” Organometallics 2020, 39, 3656–3662. DOI: 10.1021/acs.organomet.0c00398
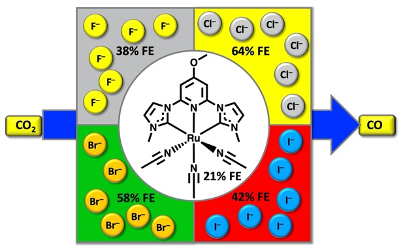
49. Shirley, H.; Figgins, M. T.*; Boudreaux, C. M.; Liyanage, N. P.; Lamb, R. W.; Webster, C. E.; Papish, E. T.; Delcamp, J. H. “Impact of the Dissolved Anion on the Electrocatalytic Reduction of CO2 to CO with Ruthenium CNC Pincer Complexes” ChemCatChem 2020, 12, 4879 – 4885. Special invited issue on “Pincer Chemistry and Catalysis.” DOI: 10.1002/cctc.202000742
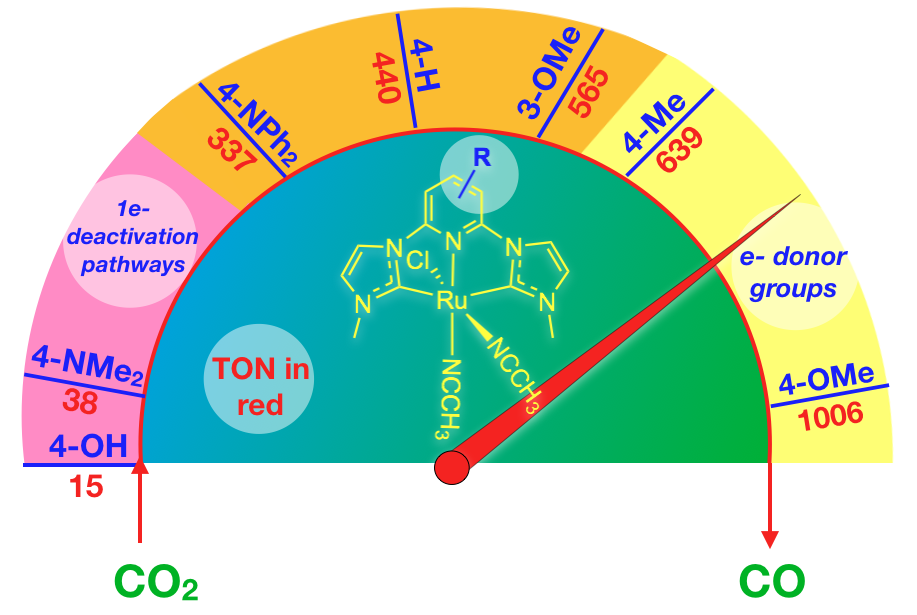
48. Das, S.; Nugegoda, D.; Qu, F.; Boudreaux, C. M.; Burrow, P. E.; Figgins, M. T.*; Lamb, R. W.; Webster, C. E.; Delcamp, J. H.; Papish, E. T. “Structure Function Relationships in Ruthenium Carbon Dioxide Reduction Catalysts with CNC Pincers Containing Donor Groups.” Eur. J. Inorg. Chem. 2020, 2709-2717. Special invited issue on “Pincer Chemistry and Catalysis,” published online 6/17/20. DOI: 10.1002/ejic.202000444
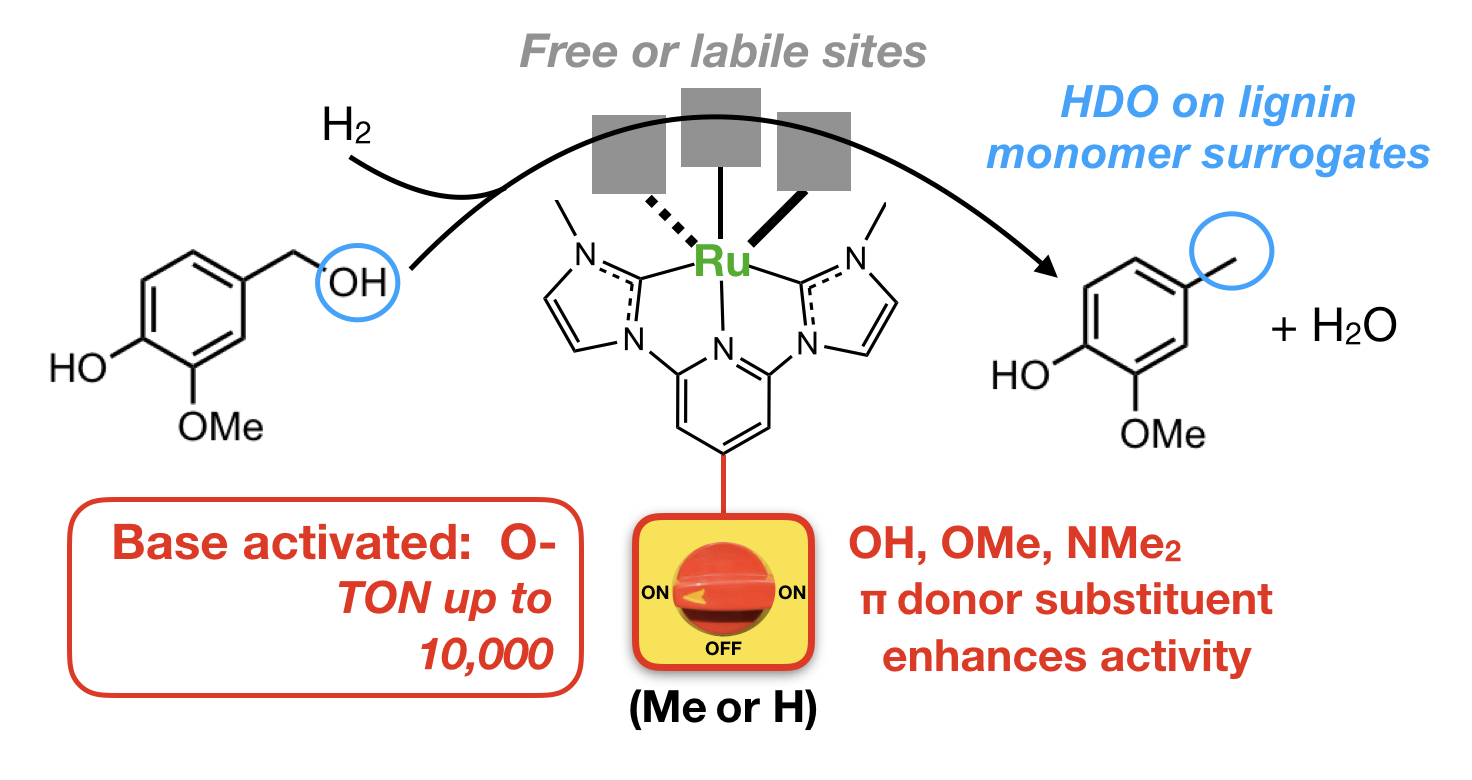
47. Yao, W.; Das. S.; DeLucia, N. A.; Qu, F.; Boudreaux, C. M.; Vannucci, A. K.; Papish, E. T. “Determining the Catalyst Properties that Lead to High Activity and Selectivity for Catalytic Hydrodeoxygenation with Ruthenium Pincer Complexes” Organometallics 2020, 39, 662-669. https://doi.org/10.1021/acs.organomet.9b00816.
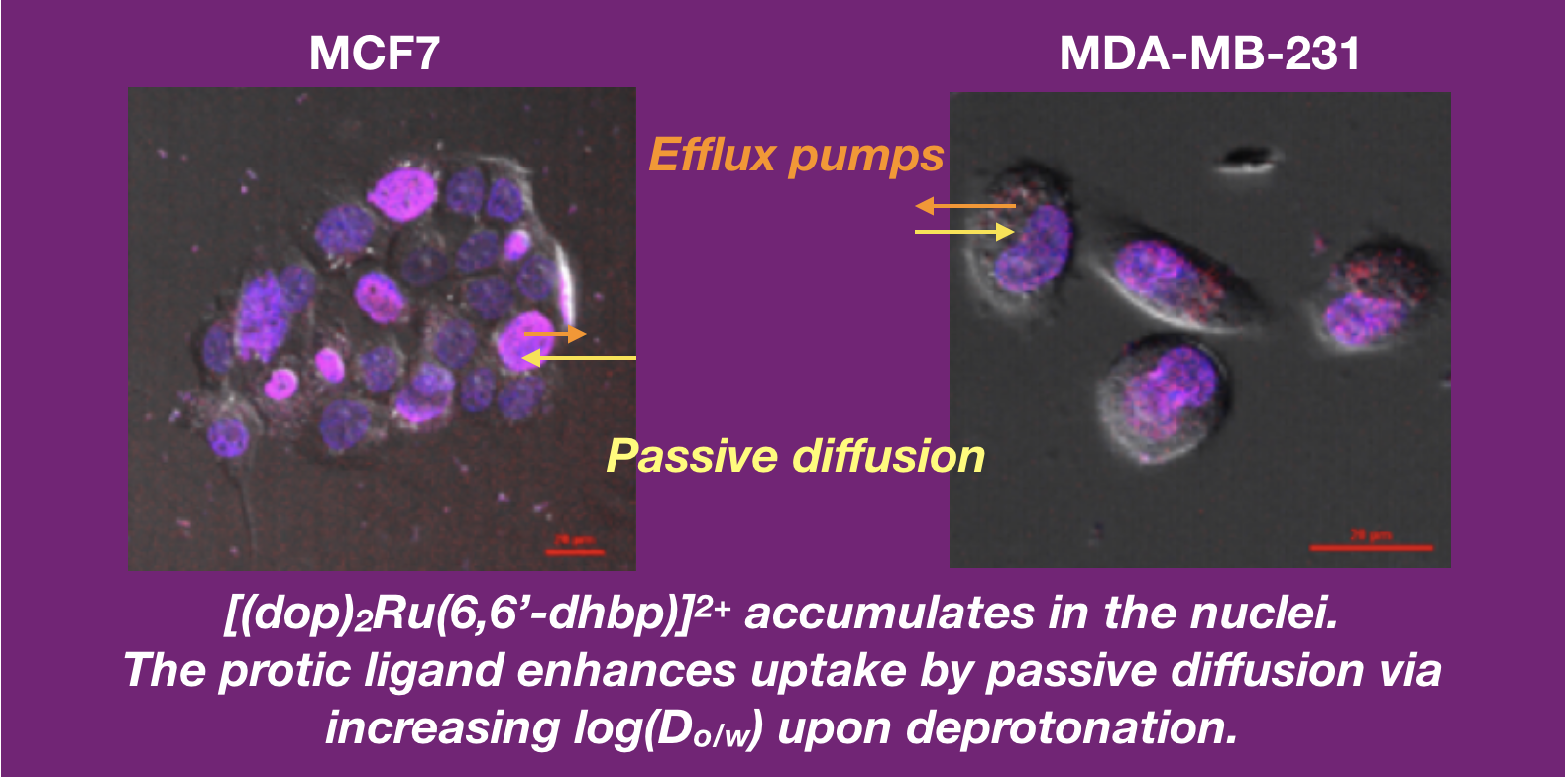
46. Park, S.; Gray, J. L.; Altman, S. D.; Hairston, A. R.; Beswick, B. T.; Kim, Y.; Papish, E. T. “Cellular Uptake of Protic Ruthenium Complexes is Influenced by pH Dependent Passive Diffusion and Energy Dependent Efflux” J. Inorg. Biochem. 2020, 203, 110922. https://doi.org/10.1016/j.jinorgbio.2019.110922.
45. Das, S.; Rodrigues, R. R.; Lamb, R. W.; Qu, F.; Reinheimer, E.; Boudreaux, C. M.; Webster, C. E.; Delcamp, J. H.; Papish, E. T. “Highly Active Ruthenium CNC Pincer Photocatalysts for Visible Light Driven Carbon Dioxide Reduction” Inorg. Chem. 2019, 58, 8012-8020. DOI: 10.1021/acs.inorgchem.9b00791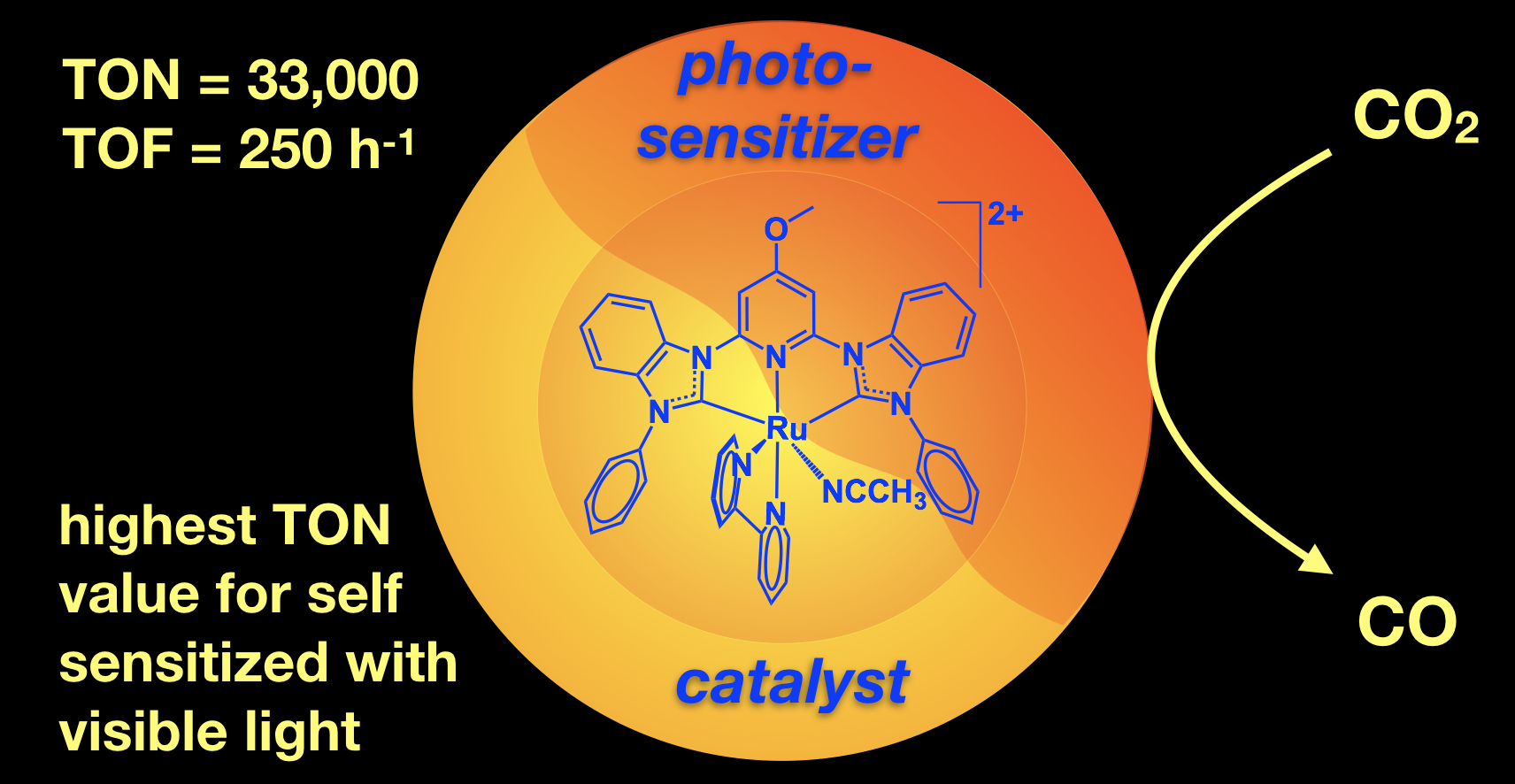
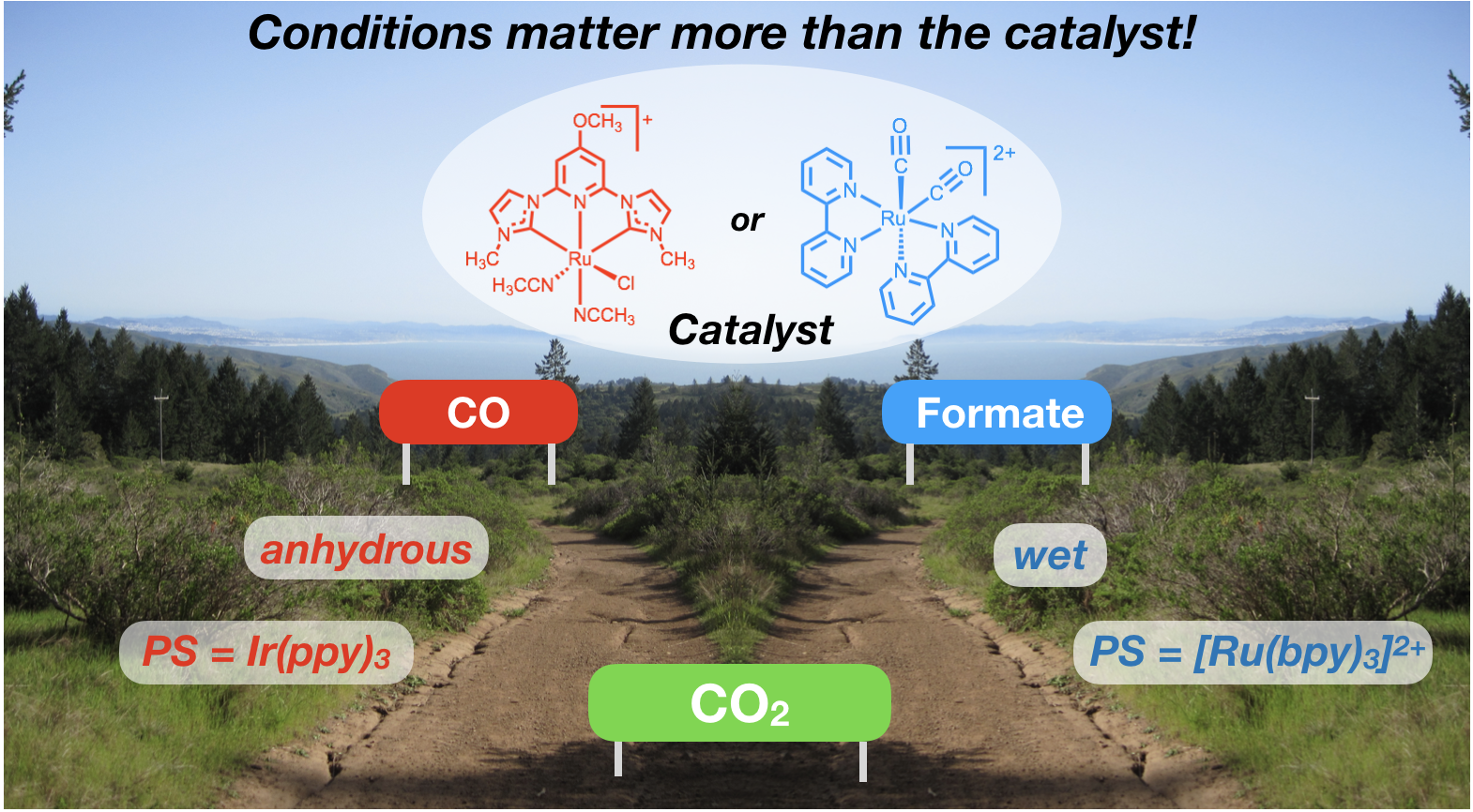
44. Rodrigues, R. R.; Boudreaux, C. M.; Papish, E. T.; Delcamp, J. H. “The Photocatalytic Reduction of CO2 to CO and Formate: Do Reaction Conditions or Ruthenium Catalysts Control Product Selectivity?”ACS Applied Energy Materials, invited forum issue on “New Chemistry to Advance the Quest for Sustainable Solar Fuels,” 2019, 2, 37-46. DOI: 10.1021/acsaem.8b01560.
43. Qu, F.; Martinez, K.; Arcidiacono, A. M.; Park, S.; Zeller, M.; Schmehl, R. H.; Paul, J. J.; Kim, Y.; Papish, E. T. “Sterically Demanding Methoxy and Methyl Groups in Ruthenium Complexes Lead to Enhanced Quantum Yields for Blue Light Triggered Photodissociation” Dalton Trans. 2018, 47, 15685-93. DOI: 10.1039/c8dt03295e.
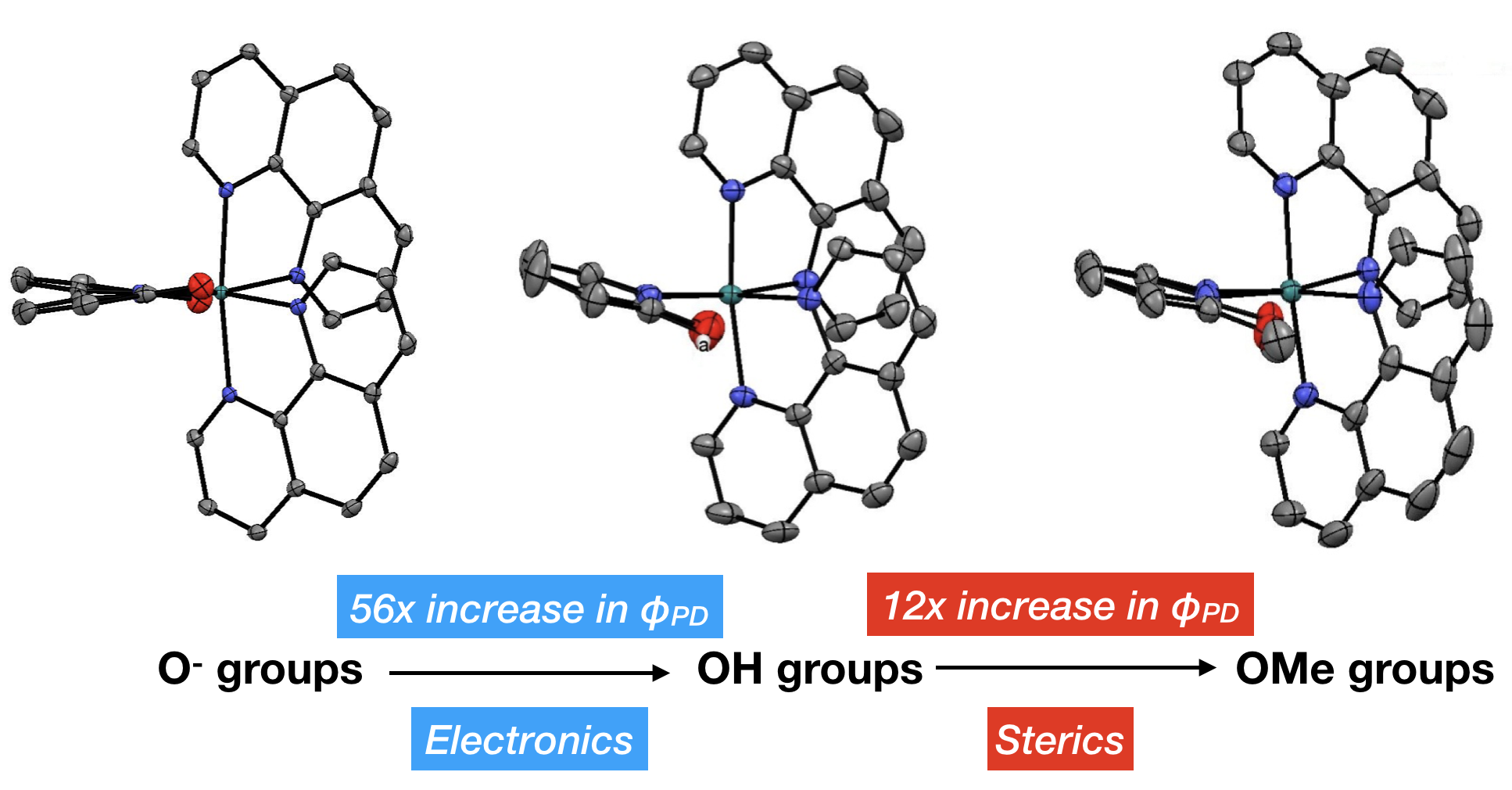
Image chosen for inside back cover of Dalton Trans.: 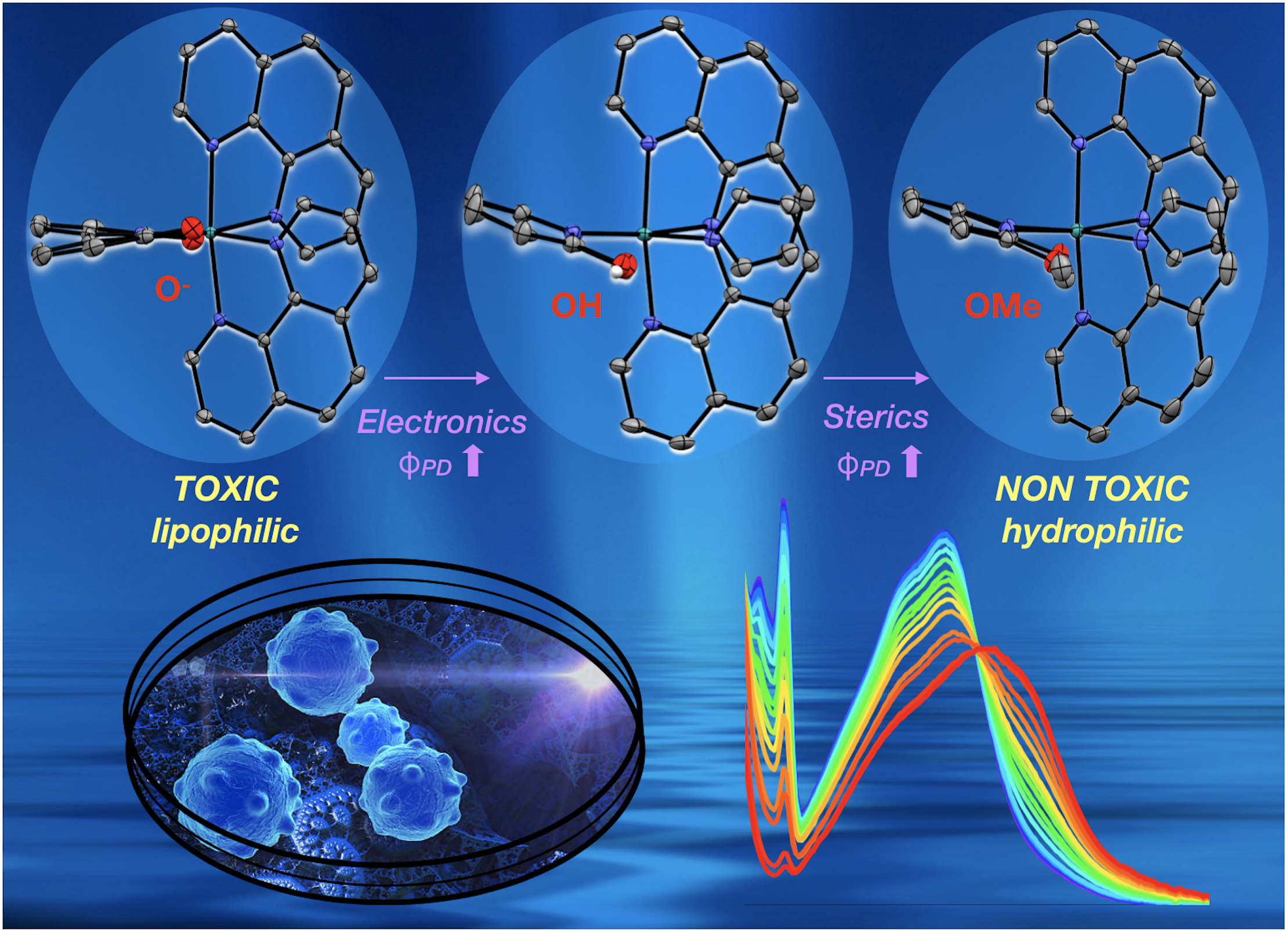
42. Lisic, E. C.; Rand, V. G.; Ngo, L.; Kent, P.; Rice, J.; Gerlach, D.; Papish, E. T.; Jiang, X., “Cu(II) Propionyl-Thiazole Thiosemicarbazone Complexes: Crystal Structure, Inhibition of Human Topoisomerase IIα, and Activity against Breast Cancer Cells.” Open Journal of Medicinal Chemistry 2018, 8, 30-46 https://doi.org/10.4236/ojmc.2018.82004
41. Burks, D. B.; Davis, S.; Lamb, R. W.; Liu, X.; Rodrigues, R. R.; Liyanage, N. P.; Sun, Y.; Webster, C. E.;* Delcamp, J. H.;* Papish, E. T.* “Nickel(II) pincer complexes demonstrate that the remote substituent controls catalytic carbon dioxide reduction” Chem. Commun. 2018, 54, 3819-3822. dx.doi.org/10.1039/c7cc09507d
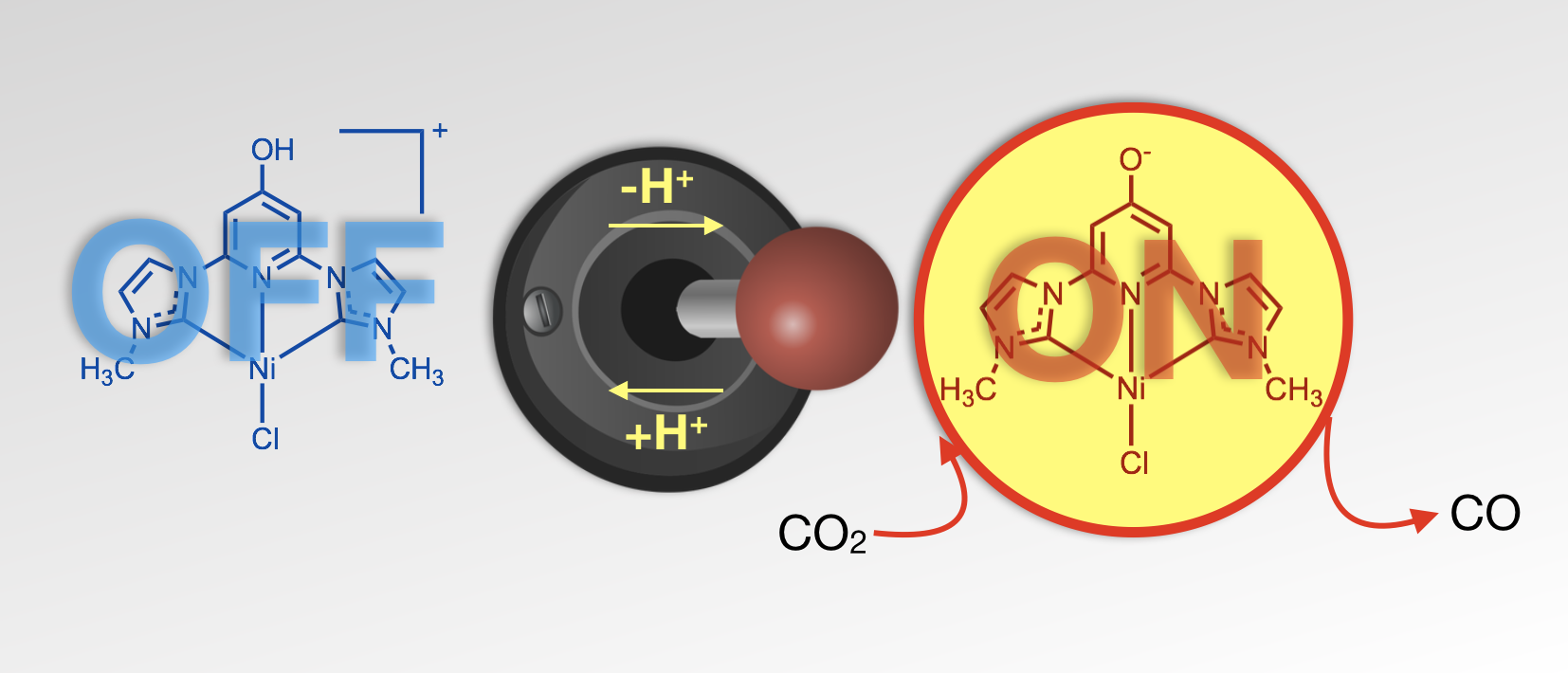
Image chosen for back cover of Chem. Commun.:
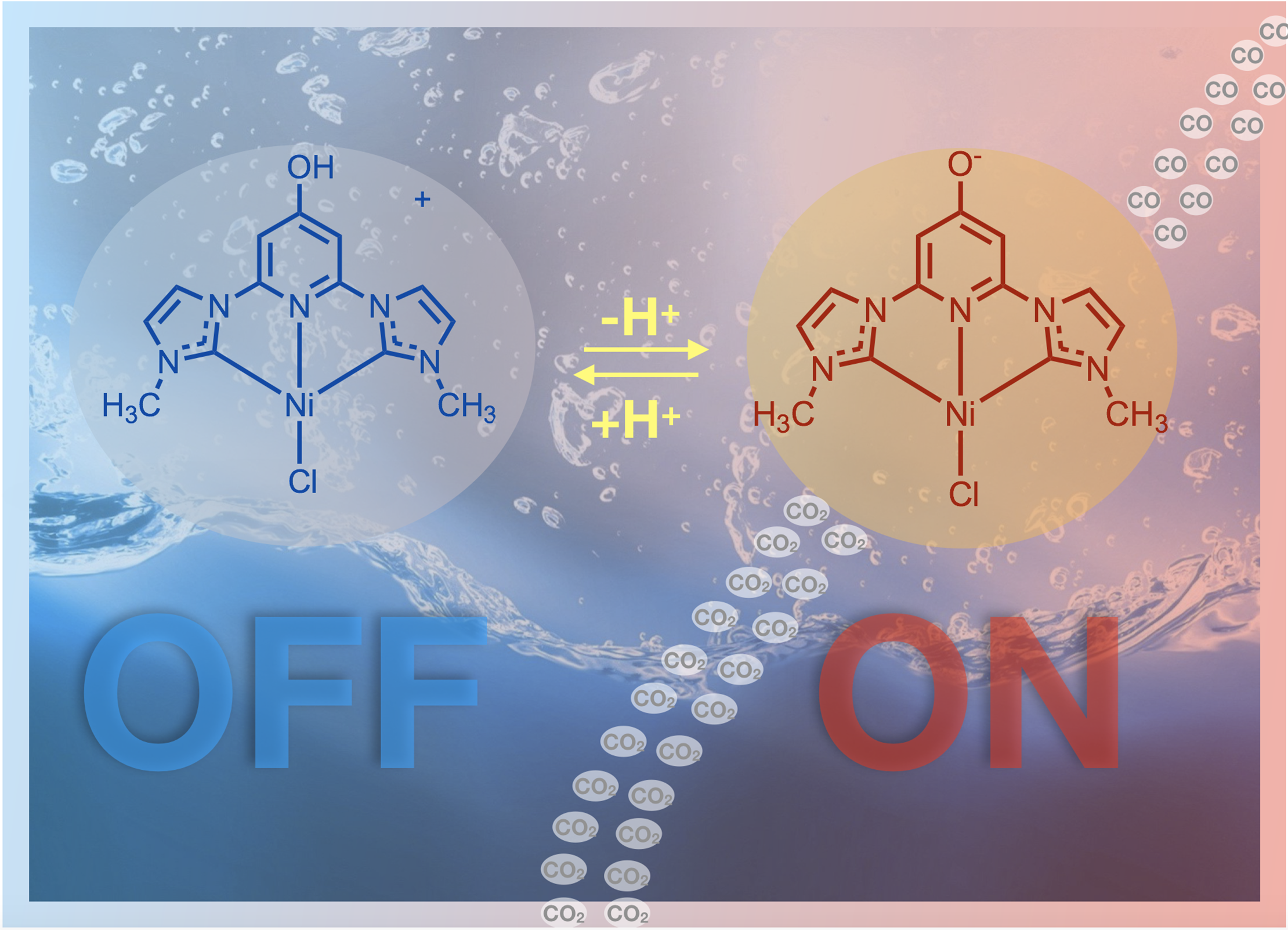
40. Burks, D. B.; Vasiliu, M.; Dixon, D. A.;* Papish, E. T.* “Thermodynamic Acidity Studies of 6,6′-dihydroxybipyridine: A Combined Experimental and Computational Approach” J. Phys. Chem. A. 2018, 122, 2221-2231. dx.doi.org/10.1021/acs.jpca.7b11441
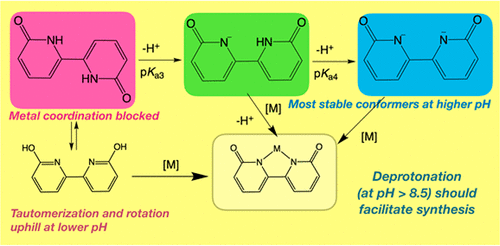
39. Boudreaux, C. M.; Liyanage, N. P.; Shirley, H.; Siek, S.; Gerlach, D. L.; Qu, F.; Delcamp, J. H.;* Papish, E. T.* “Ruthenium(II) complexes of pyridinol and N-heterocyclic carbene derived pincers as robust catalysts for selective carbon dioxide reduction” Chem. Commun. 2017, 53, 11217-11220. dx.doi.org/10.1039/c7cc05706g
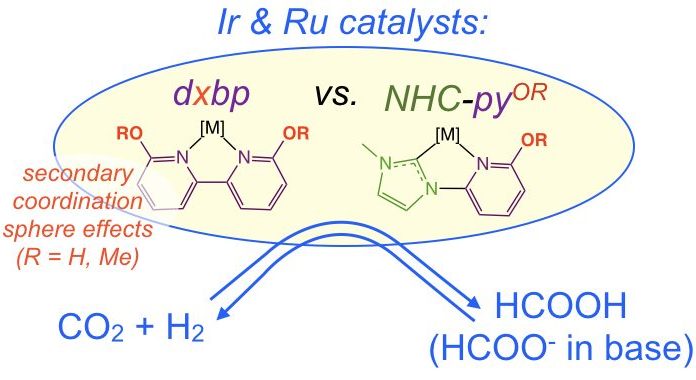
38. Gerlach, D. L.; Siek, S.; Burks, D. B.; Tesh, J. M.; Thompson, C. R.; Vasquez, R. M.; White, N. J.; Zeller, M.; Grotjahn, D. B.;* Papish, E. T.* “Ruthenium (II) and Iridium (III) Complexes of N-Heterocyclic Carbene and Pyridinol Derived Bidentate Chelates: Synthesis, Characterization, and Reactivity.” Inorganica Chimica Acta, 2017, 466, 442-450. https://doi.org/10.1016/j.ica.2017.06.063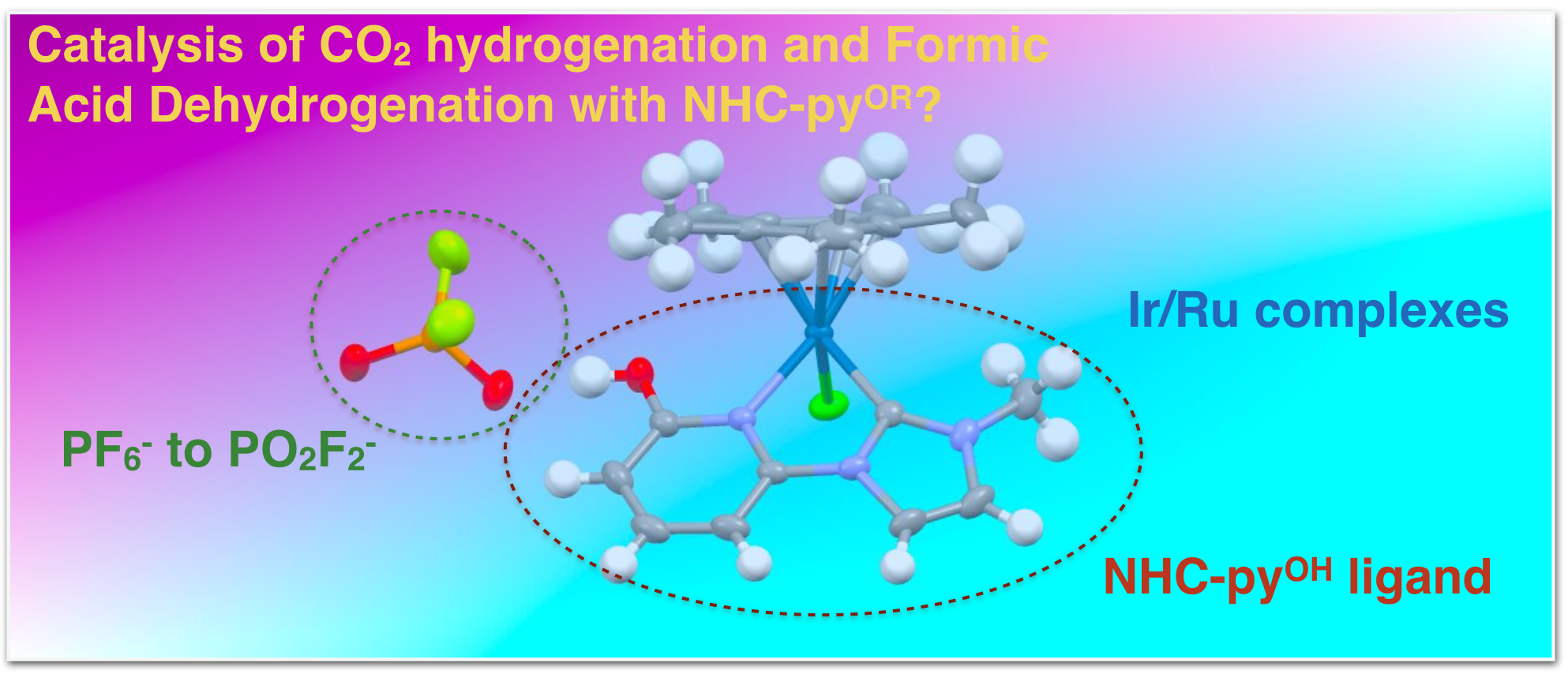
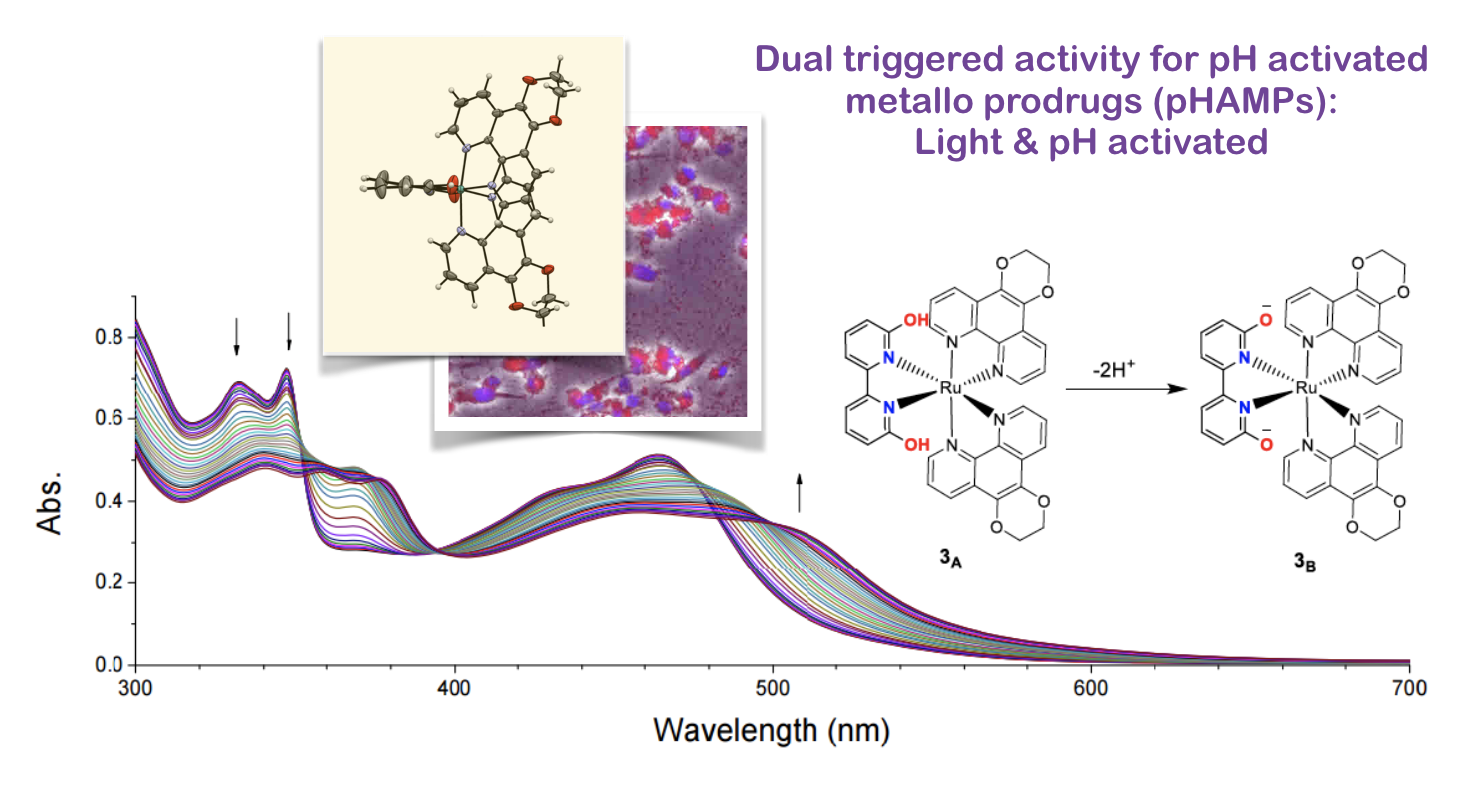
37. Qu, F.; Park, S.; Martinez, K.; Gray, J.L.; Shazna Thowfeik, F.; Lundeen, J. A.; Kuhn, A. E.; Charboneau, D. J.; Gerlach, D. L.; Lockart, M. M.; Law, J. A.; Jernigan, K. L.; Chambers, N.; Zeller, M.; Piro, N. A.; Kassel, W. S.; Schmehl, R. H.; Paul, J. J.;* Merino, E. J.;* Kim, Y.;* Papish E. T.* “Ruthenium Complexes are pH-Activated Metallo Prodrugs (pHAMPs) with Light Triggered Selective Toxicity Towards Cancer Cells” Inorganic Chemistry, 2017, 56, 7519-7532. http://dx.doi.org/10.1021/acs.inorgchem.7b01065
36. Siek, S.; Burks, D. B.; Gerlach, D. L.; Liang, G.; Tesh, J. M.; Thompson, C. R.; Qu, F.; Shankwitz, J. E.; Vasquez, R. M.; Chambers, N. S.; Szulczewski, G. J.; Grotjahn, D. B.; Webster, C. E.; Papish, E. T. “Iridium and Ruthenium Complexes of NHC and Pyridinol Derived Chelates as Catalysts for Aqueous Carbon Dioxide Hydrogenation and Formic Acid Dehydrogenation: The Role of the Alkali Metal” Organometallics, 2017, 36, 1091-1106. https://doi.org/10.1021/acs.organomet.6b00806
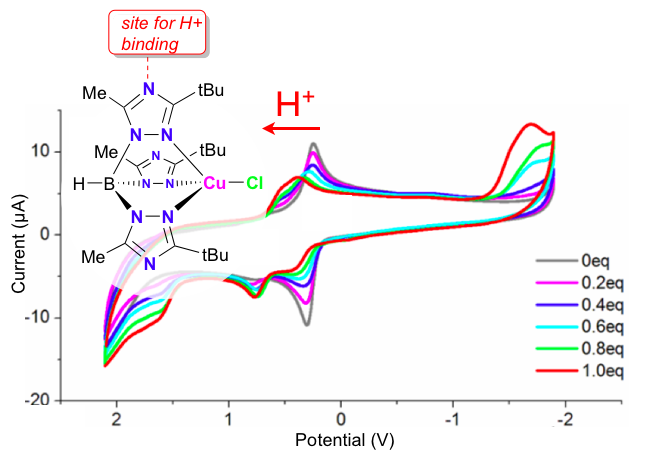
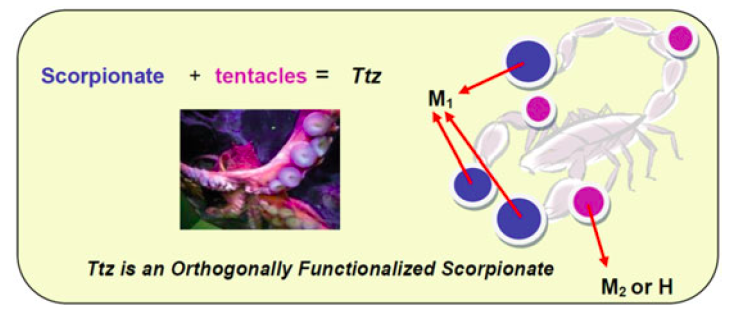
35. Siek, S.; Dixon, N. A.; Papish, E. T. “Electrochemical Reduction of Ttz Copper(II) Complexes in the Presence and Absence of Protons: Processes Relevant to Enzymatic Nitrite Reduction. (TtzR,R’ = tris(3-R, 5-R’-1, 2, 4-triazolyl)borate)” Inorganica Chimica Acta, 2017, 459, 80-86, https://doi.org/10.1016/j.ica.2017.01.021
34. Gray, J. L.; Gerlach, D. L.; Papish, E. T. “Crystal structure of [(1,4,7,10-tetraazacyclododecane)copper(II)] perchlorate” Acta Cryst. Section E: Cryst. Commun., 2017, E73, 31-34. https://doi.org/10.1107/S2056989016019563.
33. Meany, J. E.; Gerlach, D. L.; Papish, E. T.; Woski, S. A. “4′-Bromo-2′,5′-dimethoxy-2,5-dioxo-[1,1′-biphenyl]-3,4-dicarbonitrile [BrHBQ(CN)2] benzene hemisolvate”Acta Cryst. Section E: Cryst. Commun., 2016, E72, 600-603. DOI: 10.1107/S2056989016005120.
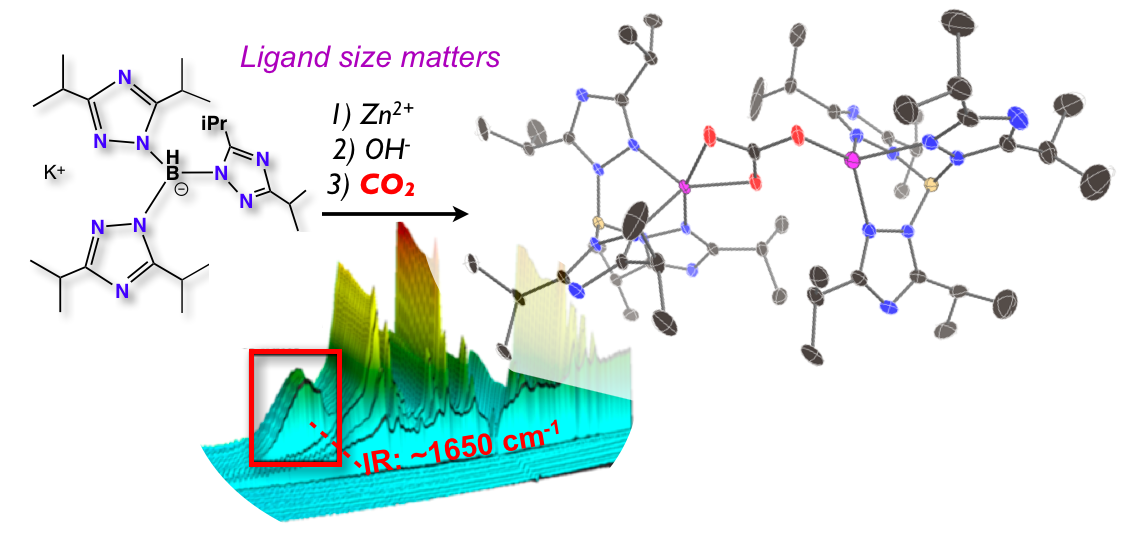
32. Siek, S.; Dixon, N. A.; Kumar, M.; Kraus, J. S.;* Wells, K. R.;* Rowe, B. W.;* Kelley, S. P.; Zeller, M.; Yap, G. P. A.; Papish, E. T. “The Synthesis of Biomimetic Zinc Complexes for CO2 Activation and the Influence of Steric Changes in the Ttz Ligands (Ttz = Tris(triazolyl)borate)” Eur. J. Inorg. Chem, 2016, 2495-2507, in Scorpionates special issue. DOI: 10.1002/ejic.201500819
31. Serpas, L.;* Baum, R. R.;* McGhee, A.;* Nieto, I.; Jernigan, K. L.;*Zeller, M.; Ferrence, G. M.; Tierney, D. L.; Papish, E. T. “‘‘Scorpionate-like” complexes that are held together by hydrogen bonds: Crystallographic and spectroscopic studies of (3-NH(t-butyl)-5-methylpyrazole)nMX2 (M = Zn, Ni, Co, Mn; n = 3, 4; X = Cl, Br)“ Polyhedron, 2016, 114, 62-71, in Undergraduate Research special issue. DOI: 10.1016/j.poly.2015.10.003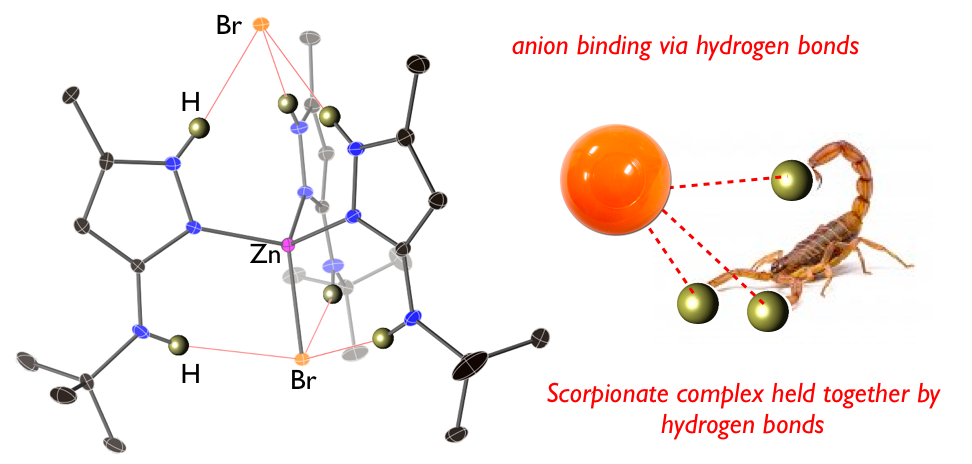
30. Gerlach, D. L.; Nieto, I.; Herbst-Gervasoni, C. J.; Ferrence, G. M.; Zeller, M.; Papish, E. T. “Crystal structures of bis- and hexakis-[copper(II)(6,6′-dihydroxybipyridine)] nitrate
coordination complexes” Acta. Cryst. 2015, E71, 1447-1453. doi:10.1107/S205698901502037X
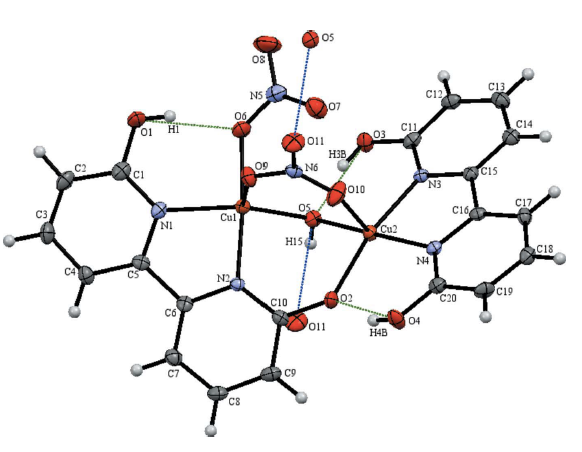
29. Gerlach, D. L.; Bhagan, S.; Cruce, A. A.; Burks, D. B.; Nieto, I.; Truong, H. T.;* Kelley, S. P.; Herbst-Gervasoni, C. J.; Jernigan, K. L.;* Bowman, M. K.; Pan, S.; Zeller, M.; Papish, E. T. “Studies of the Pathways Open to Copper Water Oxidation Catalysts Containing Proximal Hydroxy Groups During Basic Electrocatalysis” Inorganic Chemistry, 2014, 53 (24), 12689–12698. DOI: 10.1021/ic501018a.
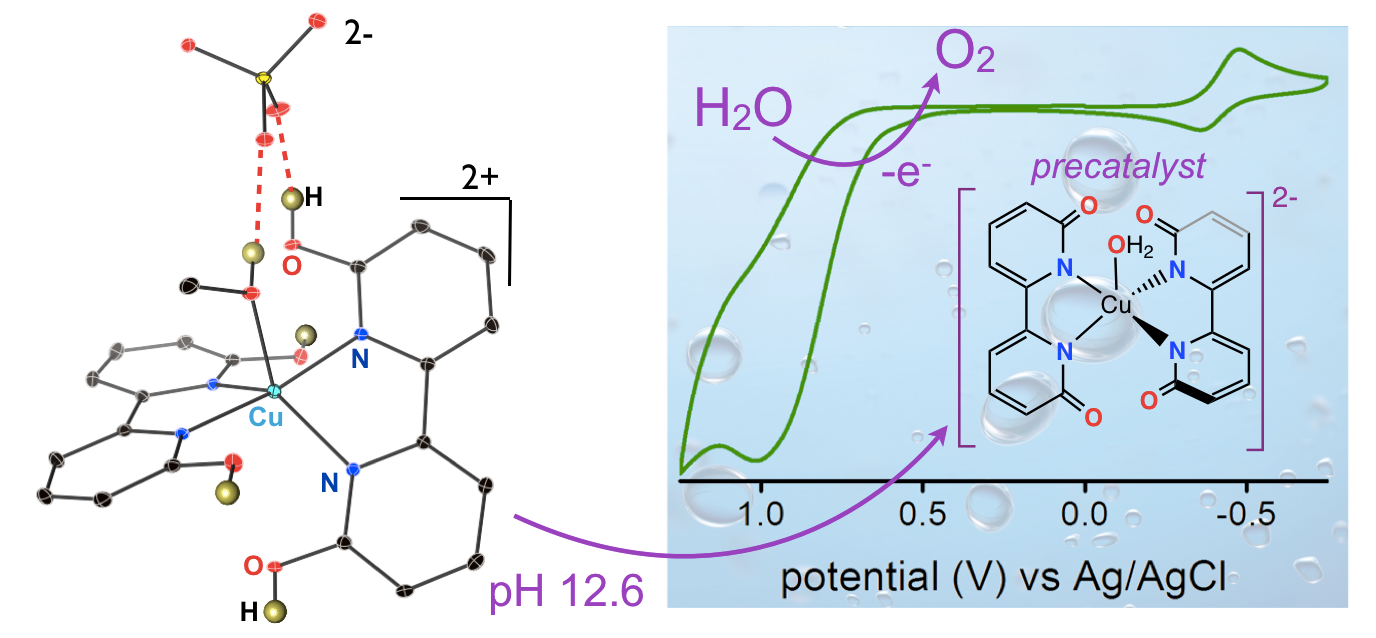
28. Marelius, D. C.; Bhagan, S.; Charboneau, D. J.; Schroeder, K. M.; Kamdar, J. M.; McGettigan, A. R.; Freeman, B. J.; Moore, C. E.; Rheingold, A. L.; Cooksy, A. L.; Smith, D. K.; Paul, J. J.; Papish, E. T.; Grotjahn, D. B. “How Do Proximal Hydroxy or Methoxy Groups on the Bidentate Ligand Affect (terpy)Ru(N,N)X Water Oxidation Catalysts? Synthesis, Characterization, and Reactivity at Acidic and Near-neutral pH” European Journal of Inorganic Chemistry, 2014, 676-689. Invited for special issue on water oxidation catalysis. DOI: 10.1002/ejic.201300826.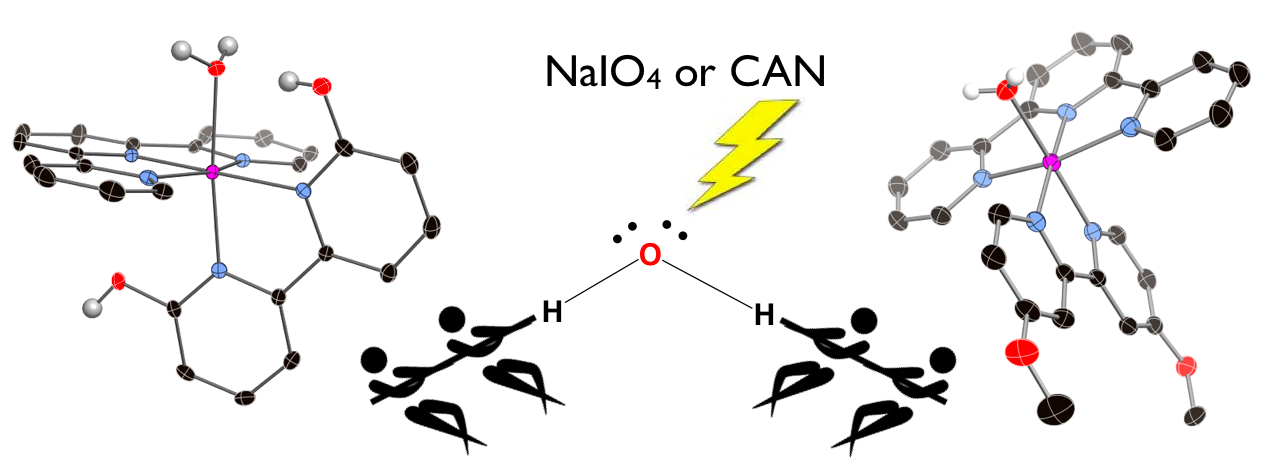
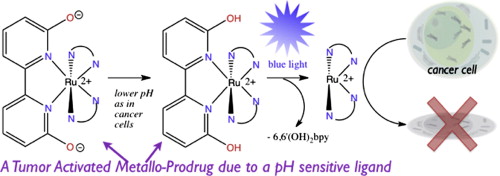
27. Hufziger,K. T.; Thowfeik, F. S.; Charboneau, D. J.; Nieto,I.; Dougherty,W. G.; Kassel, W.S; Dudley,T. J.; Merino,E. J.; Papish,E. T.; Paul, J. J. “Ruthenium Dihydroxybipyridine Complexes are Tumor Activated Prodrugs Due to Low pH and Blue Light Induced Ligand Release” Journal of Inorganic Biochemistry, 2014, 130, 103-111. DOI: 10.1016/j.jinorgbio.2013.10.008.
The following publications are from work at Drexel University:
26. Papish, E. T.; Dixon, N. A.; Kumar, M. “Biomimetic Chemistry with Tris(triazolyl)borate Ligands: Unique Structures and Reactivity via Interactions with the Remote Nitrogen” Invited review article for Structure and Bonding 2014, 160, 115-150. DOI: 10.1007/430_2012_86.
25. Dixon, N. A.; McQuarters, A. B.; Kraus, J. S.;* Soffer, J.; Lehnert, N.; Schweitzer-Stenner, R.; Papish, E. T. “Dramatic Tuning of Ligand Donor Properties in (Ttz)CuCO through Remote Binding of H+ (Ttz = tris(triazolyl)borate” Chem. Commun., 2013, 49, 5571-5573. http://pubs.rsc.org/en/Content/ArticleLanding/2013/CC/c3cc00036b
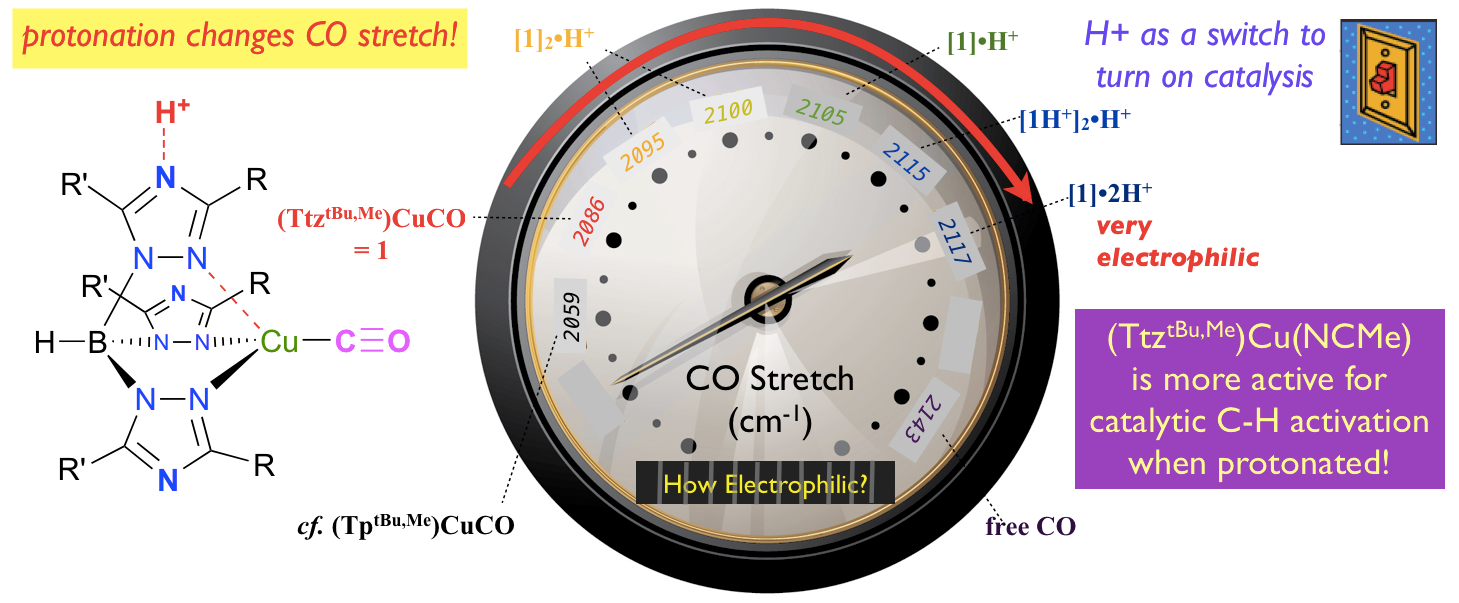
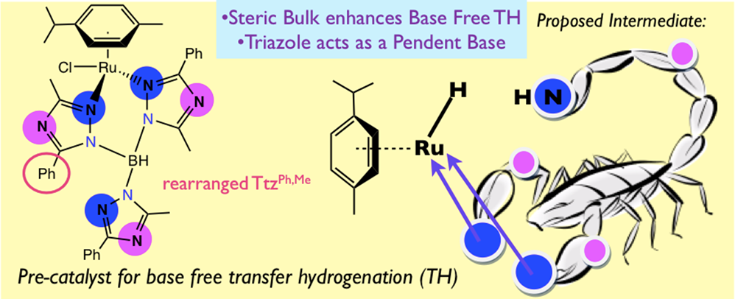
24. Kumar, M.; DePasquale, J.; White, N. J.; Zeller, M.; Papish, E. T. “Ruthenium Complexes of Triazole Based Scorpionate Ligands can Hydrogenate Substrates Under Base Free Conditions” Organometallics, 2013, 32, 2135-2144. DOI: 10.1021/om301260j.
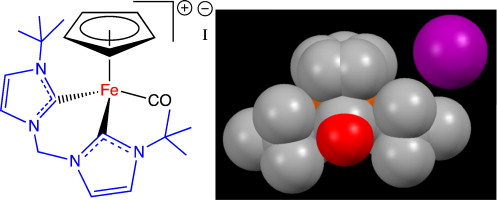
23. Kumar, M.; DePasquale, J.; Zeller, M.; Papish, E. T. “A Bulky Bis N-Heterocyclic Carbene Complex of Iron with Coordination Number of Six: [CpFe(CO)(CH2(ImtBu)2]I (where ImtBu = 3-tert-butyl-1H-imidazol-1-yl-2(3H)-ylidene)” Inorganic Chemistry Communications, 2013, 32, 55-58.
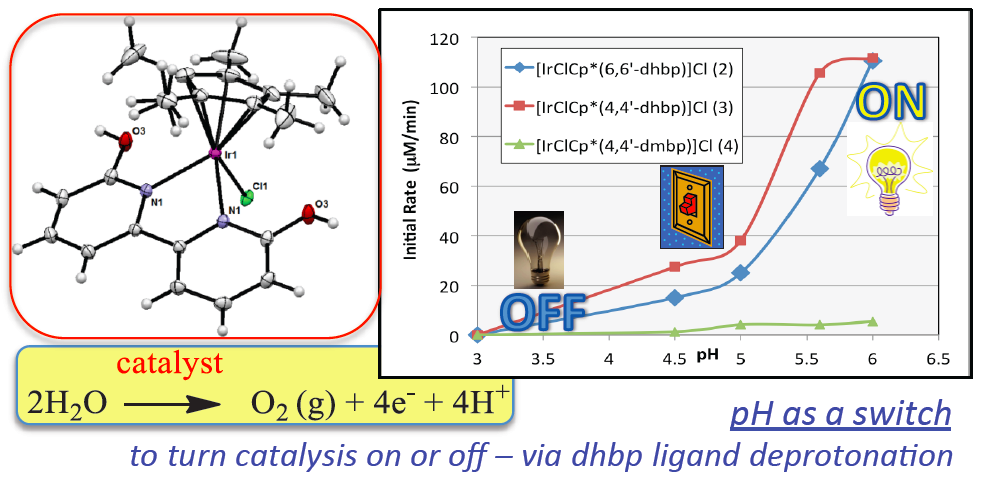
22. DePasquale, J; Nieto, I.; Reuther, L. E.;* Herbst-Gervasoni, C. J.; Paul, J. J.; Mochalin, V.; Zeller, M.; Thomas, C. M.; Addison, A. W.; Papish, E. T. “Iridium Dihydroxybiypyridine Complexes show that Ligand Deprotonation Dramatically Speeds Rates of Catalytic Water Oxidation” Inorg. Chem., 2013, 52 (16), 9175-9183, chosen for cover artwork, dx.doi.org/10.1021/ic302448d.

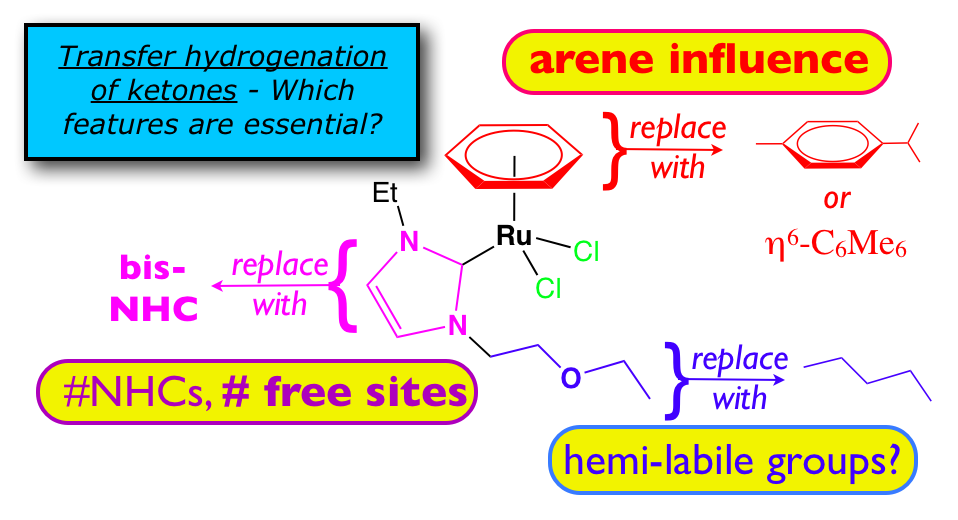
21. DePasquale, J.; Kumar, M., Zeller, M.; Papish, E. T. “Variations on an NHC Theme: Which Features are Essential for Catalysis of Transfer Hydrogenation with Ruthenium Complexes?” Organometallics, 2013, 32, 966-979. http://dx.doi.org/10.1021/om300547f.
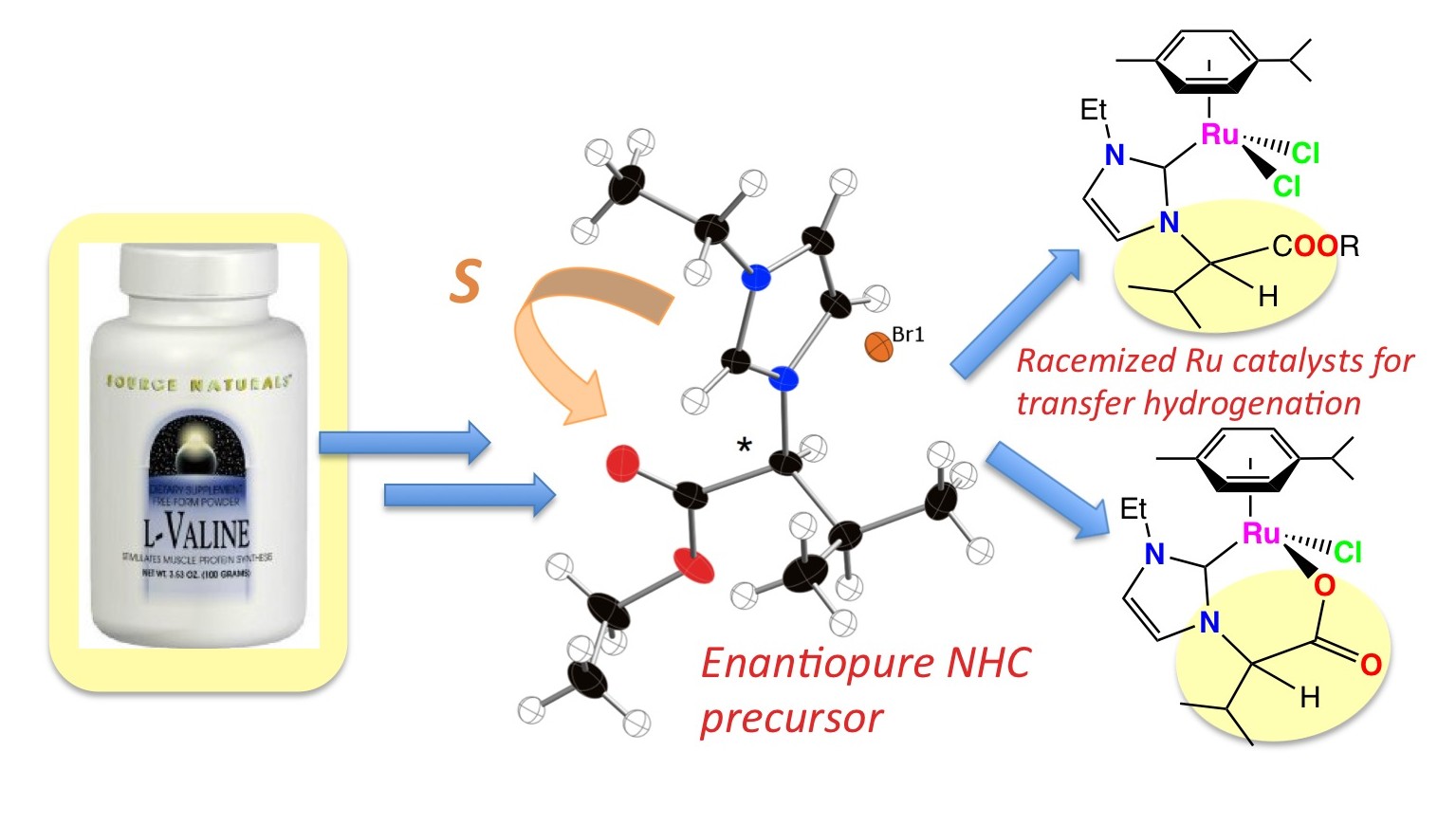
20. DePasquale, J.; White, N. J.; Ennis, E. J.; Zeller, M.; Foley, J. P.; Papish, E. T. “Synthesis of Chiral N-Heterocyclic Carbene (NHC) Ligand Precursors and Formation of Ruthenium(II) Complexes for Transfer Hydrogenation Catalysts” Polyhedron – Invited for Michelle Millar special memorial issue, 2013, 58, 162-170. DOI: http://dx.doi.org/10.1016/j.poly.2012.10.010.
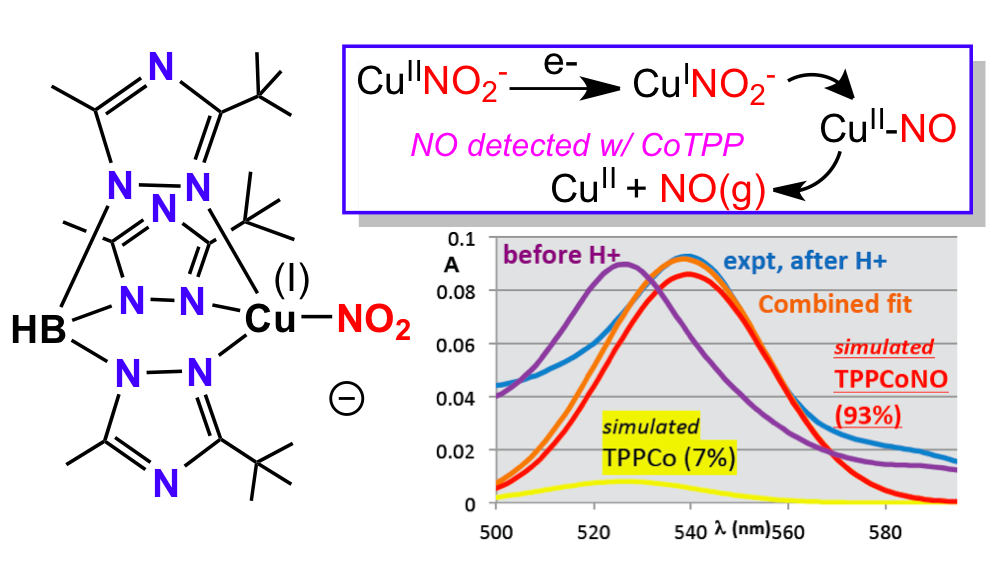
19. Kumar, M.;‡ Dixon, N. A.;‡ Merkle, A.C.; Papish, E. T.; Lehnert, N.; Zeller, M., “Hydrotris(triazolyl)borate Complexes as Functional Models for Cu Nitrite Reductase: The Electronic Influence of Distal Nitrogens.” Inorg. Chem. 2012, 7004-7006. DOI: 10.1021/ic300160c.
‡ Author Contributions The first two authors contributed equally.
18. Oseback, S. N.;*‡ Shim, S. W.;*‡ Kumar, M.;‡ Greer, S. M.; Gardner, S. R.;* Lemar, K. M.;* DeGregory, P. R.;* Papish, E. T.; Tierney, D. L.; Zeller, M.; Yap, G. P. A. “Crowded Bis Ligand Complexes of TtzPh,Me with First Row Transition Metals Rearrange due to Ligand Field Effects: Structural and Electronic Characterization (TtzPh,Me = tris(3-phenyl-5-methyl-1,2,4 triazolyl)borate)” Dalton. Trans. 2012, 41, 2774-2787. DOI: 10.1039/c2dt12029a.
‡ Author Contributions The first three authors contributed equally.
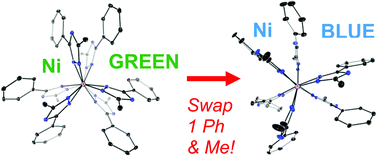
17. Nieto, I.; Livings, M. S.; Sacci, J. B. III; Reuther, L. E.;* Zeller, M.; Papish, E. T. “Transfer hydrogenation in Water via a Ruthenium Catalyst with OH Groups near the Metal Center on a Bipy Scaffold.” Organometallics 2011, 30, 6339–6342. DOI: 10.1021/om200638p
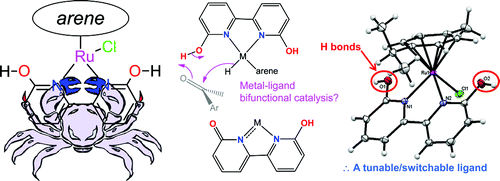
16. Kumar, M.; Papish, E. T.; Zeller, M.; Hunter, A. D. “Zinc Complexes of TtzR,Me with O and S Donors Reveal Differences Between Tp and Ttz Ligands: Acid Stability and Binding to H or an Additional Metal (TtzR,Me = tris(3-R-5-methyl-1,2,4-triazolyl)borate; R = Ph, tBu)” Dalton Trans. 2011, 40, 7517-7533. DOI: 10.1039/C1DT10429B
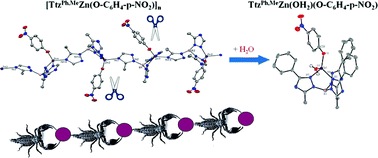
15. Marts, A. R.; Greer, S. M.; Whitehead, D. R.; Woodruff, T. M.; Breece, R. M.; Shim, S. W.;* Oseback, S. N.;* Papish, E. T.; Jacobsen, F. E.; Cohen, S. M.; Tierney, D. L. “Dual Mode EPR Studies of a Kramers ion: High-Spin Co(II) in 4-, 5- and 6-Coordination” Applied Magnetic Resonance 2011, published online April 20, 2011.
14. Kumar, M.; Papish, E. T.; Zeller, M. “Ethyl[tris(3-tert-butyl-5-methylpyrazol-1-yl)hydridoborato]zinc(II)” Acta Crystallographica Section C 2010, C66, m197-m200.
13. Bongiovanni, J. L.;* Rowe, B. W.;* Fadden, P. T.;* Taylor, M. T.;* Wells, K. R.;* Kumar, M.; Papish, E. T.; Yap, G. P. A.; Zeller, M. “Synthesis, Structural Studies and Solubility Properties of Zinc(II), Nickel(II) and Copper(II) Complexes of Bulky Tris(triazolyl)borate Ligands” Inorganica Chimica Acta 2010, 363, 2163-2170. DOI: 10.1016/j.ica.2010.03.010
12. Kumar, M.; Papish, E. T.; Zeller, M.; Hunter, A. D. “New alkylzinc complexes with bulky tris(triazolyl)borate ligands: Surprising water stability and reactivity” Dalton Trans. 2010, 39, 59-61. DOI: 10.1039/b918328k
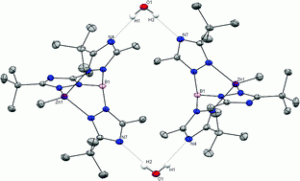
11. Papish, E. T. “A Circuitous Route to the Right Balance of Teaching and Research” In Tips on Getting an Academic Position; Pei, Z. J., Ed.; www.lulu.com: Raleigh, N.C., 2009; pp 16-23. This book chapter on mentoring future professors is available online or in paperback form. Also this can be emailed to you upon request.
10. Gardner, S. R.;* Papish, E. T.; Monillas, W. H.; Yap, G. P. A. “Tris(triazolyl)borate Ligands of Intermediate Steric Bulk for the Synthesis of Biomimetic Structures with Hydrogen Bonding and Solubility in Hydrophilic Solvents” Journal of Inorganic Biochemistry 2008, 102, 2179-2183.
9. Papish, E. T.; Donahue, T. M.;* Wells, K. R.;* Yap, G. P. A. “How Are Tris(triazolyl)borate Ligands Electronically Different From Tris(pyrazolyl)borate Ligands? A Study of (TtztBu,Me)CuCO [TtztBu,Me = tris(3-t-butyl-5-methyl-1,2,4-triazolyl)borate].” Dalton Trans. 2008, 2923-2925. DOI: 10.1039/b804951c
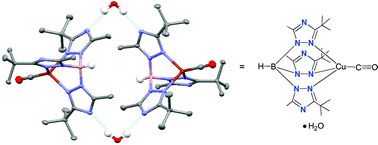
The following papers are from work at Salisbury University, a primarily undergraduate institution:
8. Jernigan, F. E.;* Sieracki, N. A.;* Taylor, M. T.;* Jenkins, A. S.;* Engel, S. E.; Rowe, B. W.;* Jové, F. A.; Yap, G. P. A.; Papish, E. T.; Ferrence, G. M. “Sterically Bulky Tris(triazolyl)borate Ligands as Water-Soluble Analogues of Tris(pyrazolyl)borate” Inorg. Chem. 2007, 46, 360-362. DOI: 10.1021/ic061828a
7. Jernigan, F. E.;* Jové, F. A.; Papish, E. T.; Yap, G. P. A. “Bis[tris(3-isopropylpyrazolyl)methane sulfonate]manganese(II)” Acta Cryst. E62, 2006, m3172-m3173.
6. Papish, Elizabeth T.; Taylor, Michael T.;* Jernigan, Finith E.;* Rodig, Michael J.;* Shawhan, Robert R.;* Yap, Glenn P. A.; Jové, Fernando A. “Synthesis of Zinc, Copper, Nickel, Cobalt and Iron Complexes Using Tris(pyrazolyl)methane Sulfonate Ligands: A Structural Model for N,N,O Binding in Metalloenzymes” Inorganic Chemistry 2006, 45, 2242-2250. DOI: 10.1021/ic051579a
The following papers are from Papish’s work as a graduate student, undergraduate student, and REU student:
5. Mahajan, Devinder; Papish, Elizabeth T.; Pandya, Kaumudi. “Sonolysis Induced Decomposition of Metal Carbonyls: Kinetics and Product Characterization.” Ultrasonics Sonochemistry 2004, 11, 385-392.
4. Tang, LiHao; Papish, Elizabeth T.; Abramo, Graham P.; Baik, Mu-Hyun; Norton, Jack R.; Rappé, Anthony. “Kinetics and Thermodynamics of H• Transfer From (h5-C5R5)Cr(CO)3H to Styrene and Methyl Methacrylate”. J. Am. Chem. Soc. 2003, 125, 10093-10102. DOI: 10.1021/ja034927l
3. Papish, Elizabeth T.; Magee, Matthew P.; Norton, Jack R. “Protonation of Transition Metal Hydrides to Give Dihydrogen Complexes: Mechanistic Implications and Catalytic Applications”. InRecent Advances in Hydride Chemistry; Poli, R., Ed.; Elsevier: Switzerland, 2001; pp 39-74.
2. Papish, Elizabeth T.; Rix, Francis C.; Spetseris, Nikos; Norton, Jack R.; Williams, Robert D. “Protonation of CpW(CO)2(PMe3)H: Is the Metal or the Hydride the Kinetic Site?” J. Am. Chem. Soc. 2000, 122, 12235-12242. DOI: 10.1021/ja002395s
1. Wu, Min; Papish, Elizabeth T.; Begley, Tadhg P. “Mechanistic studies on thiaminase I. Identification of the product of thiamin degradation in the absence of the nucleophilic cosubstrate.” Bioorg. Chem. 2000, 28, 45-48.